Abstract
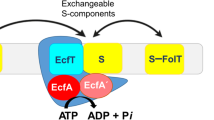
Efficient carbon utilization is critical to the survival of microorganisms in competitive environments. To optimize energy usage, bacteria have developed an integrated control system to preferentially uptake carbohydrates that support rapid growth. The availability of a preferred carbon source, such as glucose, represses the synthesis and activities of proteins necessary for the transport and metabolism of secondary carbon sources. This regulatory phenomenon is defined as carbon catabolite repression1. In enteric bacteria, the key player of carbon catabolite repression is a component of the glucose-specific phosphotransferase system, enzyme IIA (EIIAGlc)1,2. It is known that unphosphorylated EIIAGlc binds to and inhibits a variety of transporters when glucose is available1,2. However, understanding the underlying molecular mechanism has been hindered by the complete absence of structures for any EIIAGlc–transporter complexes. Here we present the 3.9 Å crystal structure of Escherichia coli EIIAGlc in complex with the maltose transporter, an ATP-binding cassette (ABC) transporter. The structure shows that two EIIAGlc molecules bind to the cytoplasmic ATPase subunits, stabilizing the transporter in an inward-facing conformation and preventing the structural rearrangements necessary for ATP hydrolysis. We also show that the half-maximal inhibitory concentrations of the full-length EIIAGlc and an amino-terminal truncation mutant differ by 60-fold, consistent with the hypothesis that the amino-terminal region, disordered in the crystal structure, functions as a membrane anchor to increase the effective EIIAGlc concentration at the membrane3,4. Together these data suggest a model of how the central regulatory protein EIIAGlc allosterically inhibits maltose uptake in E. coli.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Görke, B. & Stülke, J. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nature Rev. Microbiol. 6, 613–624 (2008)
Deutscher, J., Francke, C. & Postma, P. W. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 70, 939–1031 (2006)
Wang, G., Peterkofsky, A. & Clore, G. M. A novel membrane anchor function for the N-terminal amphipathic sequence of the signal-transducing protein IIAGlucose of the Escherichia coli phosphotransferase system. J. Biol. Chem. 275, 39811–39814 (2000)
Wang, G., Keifer, P. A. & Peterkofsky, A. Solution structure of the N-terminal amphitropic domain of Escherichia coli glucose-specific enzyme IIA in membrane-mimetic micelles. Protein Sci. 12, 1087–1096 (2003)
Hogema, B. M. et al. Inducer exclusion in Escherichia coli by non-PTS substrates: the role of the PEP to pyruvate ratio in determining the phosphorylation state of enzyme IIAGlc. Mol. Microbiol. 30, 487–498 (1998)
Khare, D., Oldham, M. L., Orelle, C., Davidson, A. L. & Chen, J. Alternating access in maltose transporter mediated by rigid-body rotations. Mol. Cell 33, 528–536 (2009)
Oldham, M. L., Khare, D., Quiocho, F. A., Davidson, A. L. & Chen, J. Crystal structure of a catalytic intermediate of the maltose transporter. Nature 450, 515–521 (2007)
Oldham, M. L. & Chen, J. Crystal structure of the maltose transporter in a pretranslocation intermediate state. Science 332, 1202–1205 (2011)
Monod, J., Wyman, J. & Changeux, J. P. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 12, 88–118 (1965)
Chen, J., Lu, G., Lin, J., Davidson, A. L. & Quiocho, F. A. A tweezers-like motion of the ATP-binding cassette dimer in an ABC transport cycle. Mol. Cell 12, 651–661 (2003)
Dean, D. A., Reizer, J., Nikaido, H. & Saier, M. H., Jr Regulation of the maltose transport system of Escherichia coli by the glucose-specific enzyme III of the phosphoenolpyruvate-sugar phosphotransferase system. Characterization of inducer exclusion-resistant mutants and reconstitution of inducer exclusion in proteoliposomes. J. Biol. Chem. 265, 21005–21010 (1990)
Kühnau, S., Reyes, M., Sievertsen, A., Shuman, H. A. & Boos, W. The activities of the Escherichia coli MalK protein in maltose transport, regulation, and inducer exclusion can be separated by mutations. J. Bacteriol. 173, 2180–2186 (1991)
Worthylake, D. et al. Three-dimensional structure of the Escherichia coli phosphocarrier protein IIIglc. Proc. Natl Acad. Sci. USA 88, 10382–10386 (1991)
Cai, M. et al. Solution structure of the phosphoryl transfer complex between the signal-transducing protein IIAGlucose and the cytoplasmic domain of the glucose transporter IICBGlucoseof the Escherichia coli glucose phosphotransferase system. J. Biol. Chem. 278, 25191–25206 (2003)
Hurley, J. H. et al. Structure of the regulatory complex of Escherichia coli IIIGlc with glycerol kinase. Science 259, 673–677 (1993)
Wang, G. et al. Solution structure of the phosphoryl transfer complex between the signal transducing proteins HPr and IIAglucose of the Escherichia coli phosphoenolpyruvate:sugar phosphotransferase system. EMBO J. 19, 5635–5649 (2000)
Pelton, J. G., Torchia, D. A., Meadow, N. D. & Roseman, S. Structural comparison of phosphorylated and unphosphorylated forms of IIIGlc, a signal-transducing protein from Escherichia coli, using three-dimensional NMR techniques. Biochemistry 31, 5215–5224 (1992)
Dörschug, M., Frank, R., Kalbitzer, H. R., Hengstenberg, W. & Deutscher, J. Phosphoenolpyruvate-dependent phosphorylation site in enzyme IIIglc of the Escherichia coli phosphotransferase system. Eur. J. Biochem. 144, 113–119 (1984)
Blschke, B., Volkmer-Engert, R. & Schneider, E. Topography of the surface of the signal-transducing protein EIIAGlc that interacts with the MalK subunits of the maltose ATP-binding cassette transporter (MalFGK2) of Salmonella typhimurium. J. Biol. Chem. 281, 12833–12840 (2006)
Stein, A. et al. Functional characterization of the maltose ATP-binding-cassette transporter of Salmonella typhimurium by means of monoclonal antibodies directed against the MalK subunit. Eur. J. Biochem. 269, 4074–4085 (2002)
Meadow, N. D. & Roseman, S. Sugar transport by the bacterial phosphotransferase system. Isolation and characterization of a glucose-specific phosphocarrier protein (IIIGlc) from Salmonella typhimurium. J. Biol. Chem. 257, 14526–14537 (1982)
Meadow, N. D., Savtchenko, R. S., Remington, S. J. & Roseman, S. Effects of mutations and truncations on the kinetic behavior of IIAGlc, a phosphocarrier and regulatory protein of the phosphoenolpyruvate phosphotransferase system of Escherichia coli. J. Biol. Chem. 281, 11450–11455 (2006)
Misko, T. P., Mitchell, W. J., Meadow, N. D. & Roseman, S. Sugar transport by the bacterial phosphotransferase system. Reconstitution of inducer exclusion in Salmonella typhimurium membrane vesicles. J. Biol. Chem. 262, 16261–16266 (1987)
van der Vlag, J., van Dam, K. & Postma, P. W. Quantification of the regulation of glycerol and maltose metabolism by IIAGlc of the phosphoenolpyruvate-dependent glucose phosphotransferase system in Salmonella typhimurium. J. Bacteriol. 176, 3518–3526 (1994)
Scholte, B. J., Schuitema, A. R. & Postma, P. W. Isolation of IIIGlc of the phosphoenolpyruvate-dependent glucose phosphotransferase system of Salmonella typhimurium. J. Bacteriol. 148, 257–264 (1981)
Kadaba, N. S., Kaiser, J. T., Johnson, E., Lee, A. & Rees, D. C. The high-affinity E. coli methionine ABC transporter: structure and allosteric regulation. Science 321, 250–253 (2008)
Gerber, S., Comellas-Bigler, M., Goetz, B. A. & Locher, K. P. Structural basis of trans-inhibition in a molybdate/tungstate ABC transporter. Science 321, 246–250 (2008)
Osumi, T. & Saier, M. H., Jr Mechanism of regulation of the lactose permease by the phosphotransferase system in Escherichia coli: evidence for protein-protein interaction. Ann. Microbiol. 133, 269–273 (1982)
Osumi, T. & Saier, M. H., Jr Regulation of lactose permease activity by the phosphoenolpyruvate:sugar phosphotransferase system: evidence for direct binding of the glucose-specific enzyme III to the lactose permease. Proc. Natl Acad. Sci. USA 79, 1457–1461 (1982)
Saier, M. H., Jr, Novotny, M. J., Comeau-Fuhrman, D., Osumi, T. & Desai, J. D. Cooperative binding of the sugar substrates and allosteric regulatory protein (enzyme IIIGlc of the phosphotransferase system) to the lactose and melibiose permeases in Escherichia coli and Salmonella typhimurium. J. Bacteriol. 155, 1351–1357 (1983)
Ujwal, S. & Bowie, J. U. Bicelle crystallization: a new method for crystallizing membrane proteins yields a monomeric bacteriorhodopsin structure. J. Mol. Biol. 316, 1–6 (2002)
Ujwal, R. & Abramson, J. High-throughput crystallization of membrane proteins using the lipidic bicelle method. J. Vis. Exp. 59, e3383 (2012)
Rossmann, M. G. The molecular replacement method. Acta Crystallogr. A 46, 73–82 (1990)
Collaborative Computational Project, 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Schröder, G. F., Levitt, M. & Brunger, A. T. Super-resolution biomolecular crystallography with low-resolution data. Nature 464, 1218–1222 (2010)
Brünger, A. T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007)
Alvarez, F. J., Orelle, C. & Davidson, A. L. Functional reconstitution of an ABC transporter in nanodiscs for use in electron paramagnetic resonance spectroscopy. J. Am. Chem. Soc. 132, 9513–9515 (2010)
Scharschmidt, B. F., Keeffe, E. B., Blankenship, N. M. & Ockner, R. K. Validation of a recording spectrophotometric method for measurement of membrane-associated Mg- and NaK-ATPase activity. J. Lab. Clin. Med. 93, 790–799 (1979)
Orelle, C., Ayvaz, T., Everly, R. M., Klug, C. S. & Davidson, A. L. Both maltose-binding protein and ATP are required for nucleotide-binding domain closure in the intact maltose ABC transporter. Proc. Natl Acad. Sci. USA 105, 12837–12842 (2008)
Acknowledgements
We thank the staff at the Advance Photon Source GM/CA-CAT, NE-CAT and SBC for assistance with data collection. This work was supported by a National Institutes of Health grant (GM070515 to J.C. and A.L.D.).
Author information
Authors and Affiliations
Contributions
All authors designed the study and analysed the data. S.C. crystallized the complex and performed the biochemical experiments. S.C. and M.L.O. determined the crystal structure and made the figures. J.C. wrote the manuscript with inputs from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-4, Supplementary Table 1 and Supplementary References. (PDF 3300 kb)
Rights and permissions
About this article
Cite this article
Chen, S., Oldham, M., Davidson, A. et al. Carbon catabolite repression of the maltose transporter revealed by X-ray crystallography. Nature 499, 364–368 (2013). https://doi.org/10.1038/nature12232
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12232
This article is cited by
-
Mechanisms of membrane protein crystallization in ‘bicelles’
Scientific Reports (2022)
-
Transporters of glucose and other carbohydrates in bacteria
Pflügers Archiv - European Journal of Physiology (2020)
-
Insights into the transcriptomic response of the plant engineering bacterium Ensifer adhaerens OV14 during transformation
Scientific Reports (2019)
-
Regulation of metabolism in Escherichia coli during growth on mixtures of the non-glucose sugars: arabinose, lactose, and xylose
Scientific Reports (2018)
-
Biodegradation of 2-hydroxyl-1,4 naphthoquinone (lawsone) by Pseudomonas taiwanensis LH-3 isolated from activated sludge
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.