Abstract
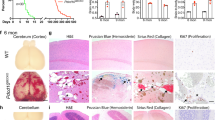
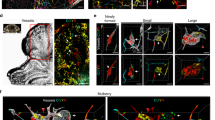
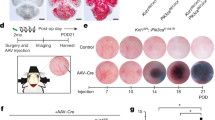
Cerebral cavernous malformation (CCM) is a vascular dysplasia, mainly localized within the brain and affecting up to 0.5% of the human population. CCM lesions are formed by enlarged and irregular blood vessels that often result in cerebral haemorrhages. CCM is caused by loss-of-function mutations in one of three genes, namely CCM1 (also known as KRIT1), CCM2 (OSM) and CCM3 (PDCD10), and occurs in both sporadic and familial forms1. Recent studies2,3,4,5,6,7 have investigated the cause of vascular dysplasia and fragility in CCM, but the in vivo functions of this ternary complex remain unclear8. Postnatal deletion of any of the three Ccm genes in mouse endothelium results in a severe phenotype, characterized by multiple brain vascular malformations that are markedly similar to human CCM lesions9. Endothelial-to-mesenchymal transition (EndMT) has been described in different pathologies, and it is defined as the acquisition of mesenchymal- and stem-cell-like characteristics by the endothelium10,11,12. Here we show that endothelial-specific disruption of the Ccm1 gene in mice induces EndMT, which contributes to the development of vascular malformations. EndMT in CCM1-ablated endothelial cells is mediated by the upregulation of endogenous BMP6 that, in turn, activates the transforming growth factor-β (TGF-β) and bone morphogenetic protein (BMP) signalling pathway. Inhibitors of the TGF-β and BMP pathway prevent EndMT both in vitro and in vivo and reduce the number and size of vascular lesions in CCM1-deficient mice. Thus, increased TGF-β and BMP signalling, and the consequent EndMT of CCM1-null endothelial cells, are crucial events in the onset and progression of CCM disease. These studies offer novel therapeutic opportunities for this severe, and so far incurable, pathology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bergametti, F. et al. Mutations within the programmed cell death 10 gene cause cerebral cavernous malformations. Am. J. Hum. Genet. 76, 42–51 (2005)
Whitehead, K. J. et al. The cerebral cavernous malformation signaling pathway promotes vascular integrity via Rho GTPases. Nature Med. 15, 177–184 (2009)
Chan, A. C. et al. Mutations in 2 distinct genetic pathways result in cerebral cavernous malformations in mice. J. Clin. Invest. 121, 1871–1881 (2011)
Glading, A., Han, J., Stockton, R. A. & Ginsberg, M. H. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell cell junctions. J. Cell Biol. 179, 247–254 (2007)
Stockton, R. A., Shenkar, R., Awad, I. A. & Ginsberg, M. H. Cerebral cavernous malformations proteins inhibit Rho kinase to stabilize vascular integrity. J. Exp. Med. 207, 881–896 (2010)
Lampugnani, M. G. et al. CCM1 regulates vascular-lumen organization by inducing endothelial polarity. J. Cell Sci. 123, 1073–1080 (2010)
Wüstehube, J. et al. Cerebral cavernous malformation protein CCM1 inhibits sprouting angiogenesis by activating DELTA-NOTCH signaling. Proc. Natl Acad. Sci. USA 107, 12640–12645 (2010)
Faurobert, E. & Albiges-Rizo, C. Recent insights into cerebral cavernous malformations: a complex jigsaw puzzle under construction. FEBS J. 277, 1084–1096 (2010)
Boulday, G. et al. Developmental timing of CCM2 loss influences cerebral cavernous malformations in mice. J. Exp. Med. 208, 1835–1847 (2011)
Zeisberg, E. M. et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nature Med. 13, 952–961 (2007)
Medici, D. et al. Conversion of vascular endothelial cells into multipotent stem-like cells. Nature Med. 16, 1400–1406 (2010)
Kalluri, R. & Weinberg, R. A. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428 (2009)
Labauge, P., Denier, C., Bergametti, F. & Tournier-Lasserve, E. Genetics of cavernous angiomas. Lancet Neurol. 6, 237–244 (2007)
Lopez, D., Niu, G., Huber, P. & Carter, W. B. Tumor-induced upregulation of Twist, Snail, and Slug represses the activity of the human VE-cadherin promoter. Arch. Biochem. Biophys. 482, 77–82 (2009)
Mariotti, A., Perotti, A., Sessa, C. & Ruegg, C. N-cadherin as a therapeutic target in cancer. Expert Opin. Investig. Drugs 16, 451–465 (2007)
Thiery, J. P., Acloque, H., Huang, R. Y. & Nieto, M. A. Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 (2009)
Li, Y., Yang, J., Luo, J. H., Dedhar, S. & Liu, Y. Tubular epithelial cell dedifferentiation is driven by the helix-loop-helix transcriptional inhibitor Id1. J. Am. Soc. Nephrol. 18, 449–460 (2007)
Vivien, C. et al. Non-viral expression of mouse Oct4, Sox2, and Klf4 transcription factors efficiently reprograms tadpole muscle fibers in vivo. J. Biol. Chem. 287, 7427–7435 (2012)
Gauger, K. J., Chenausky, K. L., Murray, M. E. & Schneider, S. S. SFRP1 reduction results in an increased sensitivity to TGF-β signaling. BMC Cancer 11, 59 (2011)
Melisi, D. et al. LY2109761, a novel transforming growth factor β receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis. Mol. Cancer Ther. 7, 829–840 (2008)
Tanaka, H. et al. Transforming growth factor beta signaling inhibitor, SB-431542, induces maturation of dendritic cells and enhances anti-tumor activity. Oncol. Rep. 24, 1637–1643 (2010)
McLean, K. et al. Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J. Clin. Invest. 121, 3206–3219 (2011)
Ao, A., Hao, J., Hopkins, C. R. & Hong, C. C. DMH1, a novel BMP small molecule inhibitor, increases cardiomyocyte progenitors and promotes cardiac differentiation in mouse embryonic stem cells. PLoS ONE 7, e41627 (2012)
Murtaugh, L. C., Stanger, B. Z., Kwan, K. M. & Melton, D. A. Notch signaling controls multiple steps of pancreatic differentiation. Proc. Natl Acad. Sci. USA 100, 14920–14925 (2003)
Korpal, M. & Kang, Y. Targeting the transforming growth factor-beta signalling pathway in metastatic cancer. Eur. J. Cancer 46, 1232–1240 (2010)
Yuan, Q. et al. Fluorofenidone suppresses epithelial-mesenchymal transition and the expression of connective tissue growth factor via inhibiting TGF-β/Smads signaling in human proximal tubular epithelial cells. Pharmazie 66, 961–967 (2011)
Glading, A. J. & Ginsberg, M. H. Rap1 and its effector KRIT1/CCM1 regulate β-catenin signaling. Dis. Model Mech. 3, 73–83 (2010)
Liebner, S. et al. β-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J. Cell Biol. 166, 359–367 (2004)
Liebner, S., Czupalla, C. J. & Wolburg, H. Current concepts of blood–brain barrier development. Int. J. Dev. Biol. 55, 467–476 (2011)
Louvi, A. et al. Loss of cerebral cavernous malformation 3 (Ccm3) in neuroglia leads to CCM and vascular pathology. Proc. Natl Acad. Sci. USA 108, 3737–3742 (2011)
Corada, M. et al. The Wnt/β-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev. Cell 18, 938–949 (2010)
Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genet. 21, 70–71 (1999)
Spagnuolo, R. et al. Gas1 is induced by VE-cadherin and vascular endothelial growth factor and inhibits endothelial cell apoptosis. Blood 103, 3005–3012 (2004)
Felici, A. et al. TLP, a novel modulator of TGF-β signaling, has opposite effects on Smad2- and Smad3-dependent signaling. EMBO J. 22, 4465–4477 (2003)
Bussolino, F. et al. Murine endothelioma cell lines transformed by polyoma middle T oncogene as target for and producers of cytokines. J. Immunol. 147, 2122–2129 (1991)
Liebner, S. et al. Wnt/β-catenin signaling controls development of the blood-brain barrier. J. Cell Biol. 183, 409–417 (2008)
Korff, T. & Augustin, H. G. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J. Cell Sci. 112, 3249–3258 (1999)
Acknowledgements
This work was supported by grants from: Fondation Leducq Transatlantic Network of Excellence, Associazione Italiana per la Ricerca sul Cancro (AIRC) and a ‘Special Program Molecular Clinical Oncology 5x1000’ to AIRC-Gruppo Italiano Malattie Mieloproliferative (AGIMM); the European Community: European Research Council (ERC grant 268870 call identifier ERC-2010-SdG) (EU Networks: EUSTROKE-contract-202213, OPTISTEM-contract-223098, ENDOSTEM-HEALTH-2009-241440, ENDOSTEM-HEALTH-2009-241440, JUSTBRAIN-HEALTH-2009-241861, ITN-VESSEL), and the CARIPLO Foundation. We are strongly indebted to R. Adams and D. A. Melton for sharing Cdh5(PAC)-CreERT2 and NotchIC mice, respectively. We are grateful to M. Forni and the Italian research network for Cerebral Cavernous Malformation (CCM Italia; http://www.ccmitalia.unito.it) for contributing to the study with important biological materials.
Author information
Authors and Affiliations
Contributions
Experiments were designed by L.M., N.R. and E.D.; in vivo treatments were performed by L.M., N.R., R.C., M.C. and L.B.; G.B. and E.T.-L. contributed to the scientific discussion and the setting-up of the in vivo experiments. Cell isolation and treatments were performed by L.M., N.R., E.P., M.G.L., C.G. and M.C.; qRT–PCR analyses were performed by L.M., N.R. and L.F.; F.O., R.C., C.R. and L.M. performed analysis of the retinas; human samples were analysed by L.M. with the help of F.C. and S.F.R.; the manuscript was assembled and written by L.M., N.R., C.G. and E.D.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-11. (PDF 4101 kb)
Rights and permissions
About this article
Cite this article
Maddaluno, L., Rudini, N., Cuttano, R. et al. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature 498, 492–496 (2013). https://doi.org/10.1038/nature12207
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12207
This article is cited by
-
Discovery and Characterization of Ephrin B2 and EphB4 Dysregulation and Novel Mutations in Cerebral Cavernous Malformations: In Vitro and Patient-Derived Evidence of Ephrin-Mediated Endothelial Cell Pathophysiology
Cellular and Molecular Neurobiology (2024)
-
Impaired retinoic acid signaling in cerebral cavernous malformations
Scientific Reports (2023)
-
Genetics of brain arteriovenous malformations and cerebral cavernous malformations
Journal of Human Genetics (2023)
-
Endothelial hyperactivation of mutant MAP3K3 induces cerebral cavernous malformation enhanced by PIK3CA GOF mutation
Angiogenesis (2023)
-
Endothelial plasticity drives aberrant vascularization and impedes cardiac repair after myocardial infarction
Nature Cardiovascular Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.