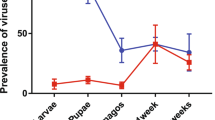
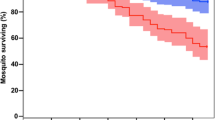
Abstract
Defining mechanisms by which Plasmodium virulence is regulated is central to understanding the pathogenesis of human malaria. Serial blood passage of Plasmodium through rodents1,2,3, primates4 or humans5 increases parasite virulence, suggesting that vector transmission regulates Plasmodium virulence within the mammalian host. In agreement, disease severity can be modified by vector transmission6,7,8, which is assumed to ‘reset’ Plasmodium to its original character3. However, direct evidence that vector transmission regulates Plasmodium virulence is lacking. Here we use mosquito transmission of serially blood passaged (SBP) Plasmodium chabaudi chabaudi9 to interrogate regulation of parasite virulence. Analysis of SBP P. c. chabaudi before and after mosquito transmission demonstrates that vector transmission intrinsically modifies the asexual blood-stage parasite, which in turn modifies the elicited mammalian immune response, which in turn attenuates parasite growth and associated pathology. Attenuated parasite virulence associates with modified expression of the pir multi-gene family. Vector transmission of Plasmodium therefore regulates gene expression of probable variant antigens in the erythrocytic cycle, modifies the elicited mammalian immune response, and thus regulates parasite virulence. These results place the mosquito at the centre of our efforts to dissect mechanisms of protective immunity to malaria for the development of an effective vaccine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dearsly, A. L., Sinden, R. E. & Self, I. A. Sexual development in malarial parasites: gametocyte production, fertility and infectivity to the mosquito vector. Parasitology 100, 359–368 (1990)
Mackinnon, M. J. & Read, A. F. Selection for high and low virulence in the malaria parasite Plasmodium chabaudi. Proc. R. Soc. Lond. B 266, 741–748 (1999)
Yoeli, M., Hargreaves, B., Carter, R. & Walliker, D. Sudden increase in virulence in a strain of Plasmodium berghei yoelii. Ann. Trop. Med. Parasitol. 69, 173–178 (1975)
Hartley, E. G. Increased virulence of Plasmodium cynomolgi bastianellii in the rhesus monkey. Trans. R. Soc. Trop. Med. Hyg. 63, 411–412 (1969)
Chin, W., Contacos, P. G., Collins, W. E., Jeter, M. H. & Alpert, E. Experimental mosquito-transmission of Plasmodium knowlesi to man and monkey. Am. J. Trop. Med. Hyg. 17, 355–358 (1968)
Alger, N. E., Branton, M., Harant, J. & Silverman, P. H. Plasmodium berghei NK65 in the inbred A-J mouse: variations in virulence of P. berghei demes. J. Protozool. 18, 598–601 (1971)
Knowles, G. & Walliker, D. Variable expression of virulence in the rodent malaria parasite Plasmodium yoelii yoelii. Parasitology 81, 211–219 (1980)
Mackinnon, M. J., Bell, A. & Read, A. F. The effects of mosquito transmission and population bottlenecking on virulence, multiplication rate and rosetting in rodent malaria. Int. J. Parasitol. 35, 145–153 (2005)
Spence, P. J., Jarra, W., Lévy, P., Nahrendorf, W. & Langhorne, J. Mosquito transmission of the rodent malaria parasite Plasmodium chabaudi. Malar. J. 11, 407 (2012)
Glynn, J. R., Collins, W. E., Jeffery, G. M. & Bradley, D. J. Infecting dose and severity of falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 89, 281–283 (1995)
Langhorne, J., Ndungu, F. M., Sponaas, A. M. & Marsh, K. Immunity to malaria: more questions than answers. Nature Immunol. 9, 725–732 (2008)
Stephens, R., Culleton, R. L. & Lamb, T. J. The contribution of Plasmodium chabaudi to our understanding of malaria. Trends Parasitol. 28, 73–82 (2012)
Spence, P. J. & Langhorne, J. T cell control of malaria pathogenesis. Curr. Opin. Immunol. 24, 444–448 (2012)
del Portillo, H. A. et al. The role of the spleen in malaria. Cell. Microbiol. 14, 343–355 (2012)
Sponaas, A. M. et al. Malaria infection changes the ability of splenic dendritic cell populations to stimulate antigen-specific T cells. J. Exp. Med. 203, 1427–1433 (2006)
Jain, V. et al. Plasma IP-10, apoptotic and angiogenic factors associated with fatal cerebral malaria in India. Malar. J. 7, 83 (2008)
Lawton, J. et al. Characterization and gene expression analysis of the cir multi-gene family of Plasmodium chabaudi chabaudi (AS). BMC Genomics 13, 125 (2012)
Cunningham, D., Lawton, J., Jarra, W., Preiser, P. & Langhorne, J. The pir multigene family of Plasmodium: antigenic variation and beyond. Mol. Biochem. Parasitol. 170, 65–73 (2010)
Brannan, L. R., McLean, S. A. & Phillips, R. S. Antigenic variants of Plasmodium chabaudi chabaudi AS and the effects of mosquito transmission. Parasite Immunol. 15, 135–141 (1993)
McLean, S. A., Phillips, R. S., Pearson, C. D. & Walliker, D. The effect of mosquito transmission of antigenic variants of Plasmodium chabaudi. Parasitology 94, 443–449 (1987)
Manske, M. et al. Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature 487, 375–379 (2012)
Peters, J. et al. High diversity and rapid changeover of expressed var genes during the acute phase of Plasmodium falciparum infections in human volunteers. Proc. Natl Acad. Sci. USA 99, 10689–10694 (2002)
Madsen, L. et al. Mice lacking all conventional MHC class II genes. Proc. Natl Acad. Sci. USA 96, 10338–10343 (1999)
Mombaerts, P. et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68, 869–877 (1992)
Chomczynski, P. & Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nature Protocols 1, 581–585 (2006)
Kyes, S., Pinches, R. & Newbold, C. A simple RNA analysis method shows var and rif multigene family expression patterns in Plasmodium falciparum. Mol. Biochem. Parasitol. 105, 311–315 (2000)
Kozarewa, I. et al. Amplification-free Illumina sequencing-library preparation facilitates improved mapping and assembly of (G+C)-biased genomes. Nature Methods 6, 291–295 (2009)
Trapnell, C., Pachter, L. & Salzberg, S. L. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111 (2009)
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome Biol. 11, R106 (2010)
Sargeant, T. J. et al. Lineage-specific expansion of proteins exported to erythrocytes in malaria parasites. Genome Biol. 7, R12 (2006)
Alexa, A., Rahnenfuhrer, J. & Lengauer, T. Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics 22, 1600–1607 (2006)
Acknowledgements
This work was supported by the Medical Research Council (U117584248) and the Wellcome Trust (089553 and 098051). P.J.S. is the recipient of a Leverhulme Trust Early Career Fellowship. The authors thank R. Sinden, K. Baker and M. Tunnicliff for provision of Anopheles stephensi, and Biological Services at NIMR. M. Blackman, G. Kassiotis and G. Stockinger are thanked for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
P.J.S., W.J. and J.L. designed the study. P.J.S., W.J., P.L., L.C. and T.B. performed the experiments. P.J.S. and A.J.R. analysed the data. M.S. and M.B. provided project management. P.J.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figure
This file contains Supplementary Figures 1-12. (PDF 1913 kb)
Supplementary Tables
This file contains Supplementary Tables 1-2. Supplementary Table 1 lists all genes identified as significantly upregulated in blood-stage parasites following mosquito transmission, whilst Supplementary Table 2 lists those upregulated following serial blood passage. (XLS 132 kb)
Rights and permissions
About this article
Cite this article
Spence, P., Jarra, W., Lévy, P. et al. Vector transmission regulates immune control of Plasmodium virulence. Nature 498, 228–231 (2013). https://doi.org/10.1038/nature12231
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12231
This article is cited by
-
Identification of gametocyte-associated pir genes in the rodent malaria parasite, Plasmodium chabaudi chabaudi AS
BMC Research Notes (2023)
-
Analysis of pir gene expression across the Plasmodium life cycle
Malaria Journal (2021)
-
Plasmodium vinckei genomes provide insights into the pan-genome and evolution of rodent malaria parasites
BMC Biology (2021)
-
The structure of a major surface antigen SAG19 from Eimeria tenella unifies the Eimeria SAG family
Communications Biology (2021)
-
Intrinsic multiplication rate variation and plasticity of human blood stage malaria parasites
Communications Biology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.