Abstract
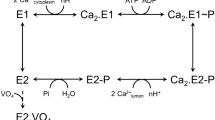
The contraction and relaxation of muscle cells is controlled by the successive rise and fall of cytosolic Ca2+, initiated by the release of Ca2+ from the sarcoplasmic reticulum and terminated by re-sequestration of Ca2+ into the sarcoplasmic reticulum as the main mechanism of Ca2+ removal. Re-sequestration requires active transport and is catalysed by the sarcoplasmic reticulum Ca2+-ATPase (SERCA), which has a key role in defining the contractile properties of skeletal and heart muscle tissue. The activity of SERCA is regulated by two small, homologous membrane proteins called phospholamban (PLB, also known as PLN) and sarcolipin (SLN)1,2. Detailed structural information explaining this regulatory mechanism has been lacking, and the structural features defining the pathway through which cytoplasmic Ca2+ enters the intramembranous binding sites of SERCA have remained unknown. Here we report the crystal structure of rabbit SERCA1a (also known as ATP2A1) in complex with SLN at 3.1 Å resolution. The regulatory SLN traps the Ca2+-ATPase in a previously undescribed E1 state, with exposure of the Ca2+ sites through an open cytoplasmic pathway stabilized by Mg2+. The structure suggests a mechanism for selective Ca2+ loading and activation of SERCA, and provides new insight into how SLN and PLB inhibition arises from stabilization of this E1 intermediate state without bound Ca2+. These findings may prove useful in studying how autoinhibitory domains of other ion pumps modulate transport across biological membranes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
James, P., Inui, M., Tada, M., Chiesi, M. & Carafoli, E. Nature and site of phospholamban regulation of the Ca2+ pump of sarcoplasmic reticulum. Nature 342, 90–92 (1989)
Odermatt, A. et al. Sarcolipin regulates the activity of SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 273, 12360–12369 (1998)
Bal, N. C. et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nature Med. 18, 1575–1579 (2012)
Asahi, M., Kimura, Y., Kurzydlowski, K., Tada, M. & MacLennan, D. H. Transmembrane helix M6 in sarco(endo)plasmic reticulum Ca2+-ATPase forms a functional interaction site with phospholamban. Evidence for physical interactions at other sites. J. Biol. Chem. 274, 32855–32862 (1999)
Asahi, M. et al. Sarcolipin regulates sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) by binding to transmembrane helices alone or in association with phospholamban. Proc. Natl Acad. Sci. USA 100, 5040–5045 (2003)
Tada, M. & Kadoma, M. Regulation of the Ca2+ pump ATPase by cAMP-dependent phosphorylation of phospholamban. Bioessays 10, 157–163 (1989)
Bhupathy, P., Babu, G. J., Ito, M. & Periasamy, M. Threonine-5 at the N-terminus can modulate sarcolipin function in cardiac myocytes. J. Mol. Cell. Cardiol. 47, 723–729 (2009)
Buffy, J. J. et al. Defining the intramembrane binding mechanism of sarcolipin to calcium ATPase using solution NMR spectroscopy. J. Mol. Biol. 358, 420–429 (2006)
Lamberth, S. et al. NMR solution structure of phosphorlamban. Helv. Chim. Acta 83, 2141–2152 (2000)
Young, H. S., Jones, L. R. & Stokes, D. L. Locating phospholamban in co-crystals with Ca2+-ATPase by cryoelectron microscopy. Biophys. J. 81, 884–894 (2001)
Møller, J. V., Olesen, C., Winther, A. M. & Nissen, P. The sarcoplasmic Ca2+-ATPase: design of a perfect chemi-osmotic pump. Q. Rev. Biophys. 43, 501–566 (2010)
Inesi, G., Ma, H., Lewis, D. & Xu, C. Ca2+ occlusion and gating function of Glu309 in the ADP-fluoroaluminate analog of the Ca2+-ATPase phosphoenzyme intermediate. J. Biol. Chem. 279, 31629–31637 (2004)
Einholm, A. P., Andersen, J. P. & Vilsen, B. Roles of transmembrane segment M1 of Na+,K+-ATPase and Ca2+-ATPase, the gatekeeper and the pivot. J. Bioenerg. Biomembr. 39, 357–366 (2007)
Dupont, Y. Low-temperature studies of the sarcoplasmic reticulum calcium pump. Mechanisms of calcium binding. Biochim. Biophys. Acta 688, 75–87 (1982)
Zhang, Z. et al. Detailed characterization of the cooperative mechanism of Ca2+ binding and catalytic activation in the Ca2+ transport (SERCA) ATPase. Biochemistry 39, 8758–8767 (2000)
Andersen, J. P. Dissection of the functional domains of the sarcoplasmic reticulum Ca2+-ATPase by site-directed mutagenesis. Biosci. Rep. 15, 243–261 (1995)
Smith, G. A., Vandenberg, J. I., Freestone, N. S. & Dixon, H. B. The effect of Mg2+ on cardiac muscle function: is CaATP the substrate for priming myofibril cross-bridge formation and Ca2+ reuptake by the sarcoplasmic reticulum? Biochem. J. 354, 539–551 (2001)
Peinelt, C. & Apell, H. J. Kinetics of the Ca2+, H+, and Mg2+ interaction with the ion-binding sites of the SR Ca-ATPase. Biophys. J. 82, 170–181 (2002)
Henderson, I. M., Starling, A. P., Wictome, M., East, J. M. & Lee, A. G. Binding of Ca2+ to the Ca2+-Mg2+-ATPase of sarcoplasmic reticulum: kinetic studies. Biochem. J. 297, 625–636 (1994)
Toyoshima, C. et al. Modeling of the inhibitory interaction of phospholamban with the Ca2+ ATPase. Proc. Natl Acad. Sci. USA 100, 467–472 (2003)
Toyofuku, T., Kurzydlowski, K., Tada, M. & MacLennan, D. H. Amino acids Glu2 to Ile18 in the cytoplasmic domain of phospholamban are essential for functional association with the Ca2+-ATPase of sarcoplasmic reticulum. J. Biol. Chem. 269, 3088–3094 (1994)
Kimura, Y., Asahi, M., Kurzydlowski, K., Tada, M. & MacLennan, D. H. Phospholamban domain Ib mutations influence functional interactions with the Ca2+-ATPase isoform of cardiac sarcoplasmic reticulum. J. Biol. Chem. 273, 14238–14241 (1998)
Chen, Z., Stokes, D. L., Rice, W. J. & Jones, L. R. Spatial and dynamic interactions between phospholamban and the canine cardiac Ca2+ pump revealed with use of heterobifunctional cross-linking agents. J. Biol. Chem. 278, 48348–48356 (2003)
Mascioni, A., Karim, C., Barany, G., Thomas, D. D. & Veglia, G. Structure and orientation of sarcolipin in lipid environments. Biochemistry 41, 475–482 (2002)
Kimura, Y., Kurzydlowski, K., Tada, M. & MacLennan, D. H. Phospholamban inhibitory function is activated by depolymerization. J. Biol. Chem. 272, 15061–15064 (1997)
Glaves, J. P., Trieber, C. A., Ceholski, D. K., Stokes, D. L. & Young, H. S. Phosphorylation and mutation of phospholamban alter physical interactions with the sarcoplasmic reticulum calcium pump. J. Mol. Biol. 405, 707–723 (2011)
Di Leva, F., Domi, T., Fedrizzi, L., Lim, D. & Carafoli, E. The plasma membrane Ca2+ ATPase of animal cells: structure, function and regulation. Arch. Biochem. Biophys. 476, 65–74 (2008)
Tidow, H. et al. A bimodular mechanism of calcium control in eukaryotes. Nature 491, 468–472 (2012)
Wu, C. C., Rice, W. J. & Stokes, D. L. Structure of a copper pump suggests a regulatory role for its metal-binding domain. Structure 16, 976–985 (2008)
Füzesi, M. et al. Covalent cross-links between the gamma subunit (FXYD2) and α and β subunits of Na,K-ATPase: modeling the α-γ interaction. J. Biol. Chem. 280, 18291–18301 (2005)
Andersen, J. P., Lassen, K. & Møller, J. V. Changes in Ca2+ affinity related to conformational transitions in the phosphorylated state of soluble monomeric Ca2+-ATPase from sarcoplasmic reticulum. J. Biol. Chem. 260, 371–380 (1985)
Kabsch, W. XDS. Acta Crystallogr. D 66, 125–132 (2010)
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007)
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Arendall, W. B. et al. A test of enhancing model accuracy in high-throughput crystallography. J. Struct. Funct. Genomics 6, 1–11 (2005)
Toyoshima, C., Nakasako, M., Nomura, H. & Ogawa, H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature 405, 647–655 (2000)
Toyoshima, C. & Nomura, H. Structural changes in the calcium pump accompanying the dissociation of calcium. Nature 418, 605–611 (2002)
Sørensen, T. L., Moller, J. V. & Nissen, P. Phosphoryl transfer and calcium ion occlusion in the calcium pump. Science 304, 1672–1675 (2004)
Toyoshima, C. & Mizutani, T. Crystal structure of the calcium pump with a bound ATP analogue. Nature 430, 529–535 (2004)
Olesen, C., Sorensen, T. L., Nielsen, R. C., Moller, J. V. & Nissen, P. Dephosphorylation of the calcium pump coupled to counterion occlusion. Science 306, 2251–2255 (2004)
Toyoshima, C., Nomura, H. & Tsuda, T. Lumenal gating mechanism revealed in calcium pump crystal structures with phosphate analogues. Nature 432, 361–368 (2004)
Jensen, A. M., Sorensen, T. L., Olesen, C., Moller, J. V. & Nissen, P. Modulatory and catalytic modes of ATP binding by the calcium pump. EMBO J. 25, 2305–2314 (2006)
Olesen, C. et al. The structural basis of calcium transport by the calcium pump. Nature 450, 1036–1042 (2007)
Laursen, M. et al. Cyclopiazonic acid is complexed to a divalent metal ion when bound to the sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 284, 13513–13518 (2009)
Toyoshima, C. How Ca2+-ATPase pumps ions across the sarcoplasmic reticulum membrane. Biochim. Biophys. Acta 1793, 941–946 (2009)
Gourdon, P. et al. Crystal structure of a copper-transporting PIB-type ATPase. Nature 475, 59–64 (2011)
Morth, J. P. et al. Crystal structure of the sodium-potassium pump. Nature 450, 1043–1049 (2007)
Acknowledgements
We thank the staff at beamlines 911-3 at MaxLab and X06SA at the Swiss Light Source. We are grateful to N. Høgholm Jonassen, C. Olesen, B. Nielsen and A.-M. Nielsen for assistance with experimental procedures, and to H. Tidow and P. Gourdon for discussions on autoinhibition. We also thank P. Vangheluwe, H. Young, M. le Maire and E. Carafoli for discussions on PLB and SLN. A.-M.L.W. was supported by postdoctoral fellowships from the Danish Council for Independent Research (Technology and Production Sciences) and the Danish National Advanced Technology Foundation. P.N. is supported by an ERC advanced research grant (BIOMEMOS). Further support was obtained from the Fungalfight and Spotlight projects of the Danish Research Council for Strategic Research.
Author information
Authors and Affiliations
Contributions
A.-M.L.W., J.B.H. and M.J.B.-P. initiated the project. J.V.M. provided sarcoplasmic reticulum membranes and purified SERCA1a, and A.-M.L.W. performed crystallization experiments. Data collection, structure determination and model refinement were performed by M.B. and A.-M.L.W. Videos were prepared by J.L.K. and A.-M.L.W. Model analysis and interpretation was done by M.B., P.N., A.-M.L.W., J.L.K., J.V.M. and M.J.B.-P. The paper was written by M.B., A.-M.L.W., M.J.B.-P. and P.N. with comments from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-12, which provide detailed close-up views and highlight different aspects of the SERCA–SLN complex structure that are described in detail in the main article and Supplementary Tables 1-3, which list the crystallographic data statistics and the residues in the SERCA–SLN interface with their TM helix assignments and conservation scores. (PDF 2510 kb)
Details of the SERCA–SLN complex structure
This video highlights the novel conformation of the SERCA-SLN complex, particularly at the ion binding site region and at the SERCA-SLN interface. (MOV 27728 kb)
Conformational changes of SERCA along the catalytic cycle
Morphing simulation between structures of the different conformational states of SERCA. (SLN and Mg2+ have been omitted for clarity.) The animation shows the 'sliding door' movement of the kinked M1, which leads to the opening of the Ca2+ entry pathway. Upon closure of the entry pathway and Ca2+-occlusion, contacts between the N- and A-domain are formed by a movement of the N-domain towards the P-domain and a rotation of the A-domain. (MOV 28436 kb)
Model of SLN regulation of SERCA
Morphing simulation of SLN binding to different conformational states of SERCA, assuming that binding is possible in the [Hn]E2 state, but not in [Ca2]E1P. An exchange process of Mg2+ with Ca2+ provides a putative structural basis for a modulatory function of Mg2+ on SERCA activity at high Mg2+ concentrations. (MOV 27353 kb)
Rights and permissions
About this article
Cite this article
Winther, AM., Bublitz, M., Karlsen, J. et al. The sarcolipin-bound calcium pump stabilizes calcium sites exposed to the cytoplasm. Nature 495, 265–269 (2013). https://doi.org/10.1038/nature11900
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11900
This article is cited by
-
Electrostatic interactions between single arginine and phospholipids modulate physiological properties of sarcoplasmic reticulum Ca2+-ATPase
Scientific Reports (2022)
-
Structural basis of ion uptake in copper-transporting P1B-type ATPases
Nature Communications (2022)
-
Targeting oncogenic Notch signaling with SERCA inhibitors
Journal of Hematology & Oncology (2021)
-
Sarcolipin alters SERCA1a interdomain communication by impairing binding of both calcium and ATP
Scientific Reports (2021)
-
The orphan solute carrier SLC10A7 is a novel negative regulator of intracellular calcium signaling
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.