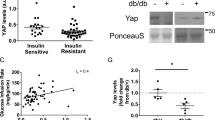
Abstract
Insulin resistance is a fundamental pathogenic factor present in various metabolic disorders including obesity and type 2 diabetes1. Although skeletal muscle accounts for 70–90% of insulin-stimulated glucose disposal2,3, the mechanism underlying muscle insulin resistance is poorly understood. Here we show in mice that muscle-specific mitsugumin 53 (MG53; also called TRIM72) mediates the degradation of the insulin receptor and insulin receptor substrate 1 (IRS1), and when upregulated, causes metabolic syndrome featuring insulin resistance, obesity, hypertension and dyslipidaemia. MG53 expression is markedly elevated in models of insulin resistance, and MG53 overexpression suffices to trigger muscle insulin resistance and metabolic syndrome sequentially. Conversely, ablation of MG53 prevents diet-induced metabolic syndrome by preserving the insulin receptor, IRS1 and insulin signalling integrity. Mechanistically, MG53 acts as an E3 ligase targeting the insulin receptor and IRS1 for ubiquitin-dependent degradation, comprising a central mechanism controlling insulin signal strength in skeletal muscle. These findings define MG53 as a novel therapeutic target for treating metabolic disorders and associated cardiovascular complications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Eckel, R. H., Grundy, S. M. & Zimmet, P. Z. The metabolic syndrome. Lancet 365, 1415–1428 (2005)
DeFronzo, R. A. et al. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30, 1000–1007 (1981)
Shulman, G. I. et al. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N. Engl. J. Med. 322, 223–228 (1990)
McMillen, I. C. & Robinson, J. S. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol. Rev. 85, 571–633 (2005)
Grundy, S. M. Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. J. Am. Coll. Cardiol. 47, 1093–1100 (2006)
DeFronzo, R. A. & Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 32 (Suppl. 2). S157–S163 (2009)
Lillioja, S. et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N. Engl. J. Med. 329, 1988–1992 (1993)
Martin, B. C. et al. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet 340, 925–929 (1992)
Cai, C. et al. MG53 nucleates assembly of cell membrane repair machinery. Nature Cell Biol. 11, 56–64 (2009)
Cao, C. M. et al. MG53 constitutes a primary determinant of cardiac ischemic preconditioning. Circulation 121, 2565–2574 (2010)
Fu, J. et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α. Nature 425, 90–93 (2003)
Rao, R. H. Insulin resistance in spontaneously hypertensive rats. Difference in interpretation based on insulin infusion rate or on plasma insulin in glucose clamp studies. Diabetes 42, 1364–1371 (1993)
Zhang, X. et al. Rhesus macaques develop metabolic syndrome with reversible vascular dysfunction responsive to pioglitazone. Circulation 124, 77–86 (2011)
Coleman, D. L. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia 14, 141–148 (1978)
Saltiel, A. R. & Kahn, C. R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799–806 (2001)
Thirone, A. C., Huang, C. & Klip, A. Tissue-specific roles of IRS proteins in insulin signaling and glucose transport. Trends Endocrinol. Metab. 17, 72–78 (2006)
Kerouz, N. J., Horsch, D., Pons, S. & Kahn, C. R. Differential regulation of insulin receptor substrates-1 and -2 (IRS-1 and IRS-2) and phosphatidylinositol 3-kinase isoforms in liver and muscle of the obese diabetic (ob/ob) mouse. J. Clin. Invest. 100, 3164–3172 (1997)
Goodyear, L. J. et al. Insulin receptor phosphorylation, insulin receptor substrate-1 phosphorylation, and phosphatidylinositol 3-kinase activity are decreased in intact skeletal muscle strips from obese subjects. J. Clin. Invest. 95, 2195–2204 (1995)
Olefsky, J., Bacon, V. C. & Baur, S. Insulin receptors of skeletal muscle: specific insulin binding sites and demonstration of decreased numbers of sites in obese rats. Metabolism 25, 179–191 (1976)
Capozza, F. et al. Caveolin-3 knockout mice show increased adiposity and whole body insulin resistance, with ligand-induced insulin receptor instability in skeletal muscle. Am. J. Physiol. Cell Physiol. 288, C1317–C1331 (2005)
Zisman, A. et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nature Med. 6, 924–928 (2000)
Brüning, J. C. et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol. Cell 2, 559–569 (1998)
Laustsen, P. G. et al. Essential role of insulin and insulin-like growth factor 1 receptor signaling in cardiac development and function. Mol. Cell. Biol. 27, 1649–1664 (2007)
Nakao, R. et al. Ubiquitin ligase Cbl-b is a negative regulator for insulin-like growth factor 1 signaling during muscle atrophy caused by unloading. Mol. Cell. Biol. 29, 4798–4811 (2009)
Ramos, F. J., Langlais, P. R., Hu, D., Dong, L. Q. & Liu, F. Grb10 mediates insulin-stimulated degradation of the insulin receptor: a mechanism of negative regulation. Am. J. Physiol. Endocrinol. Metab. 290, E1262–E1266 (2006)
Rui, L., Yuan, M., Frantz, D., Shoelson, S. & White, M. F. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J. Biol. Chem. 277, 42394–42398 (2002)
Xu, X. et al. The CUL7 E3 ubiquitin ligase targets insulin receptor substrate 1 for ubiquitin-dependent degradation. Mol. Cell 30, 403–414 (2008)
Shi, J., Luo, L., Eash, J., Ibebunjo, C. & Glass, D. J. The SCF-Fbxo40 complex induces IRS1 ubiquitination in skeletal muscle, limiting IGF1 signaling. Dev. Cell 21, 835–847 (2011)
Joazeiro, C. A. et al. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science 286, 309–312 (1999)
Huang, H. et al. Profiling of mismatch discrimination in RNAi enabled rational design of allele-specific siRNAs. Nucleic Acids Res. 37, 7560–7569 (2009)
Zhong, L. et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1α. Cell 140, 280–293 (2010)
Acknowledgements
We thank H. P. Cheng, G. Feng, X. Fu and L. P. Wei for discussions, and S. L. Guo, T. Zhang, X. H. Wang, D. Y. Chen, J. Y. Peng, L. Huang, W. Q. Zhang, N. Hou, L. Pan, L. Chen and Y. L. Liu for their technical support. Special thanks to H. Takeshima and J. J. Ma for their support in providing MG53−/− mice. This work was supported by the National Basic Research Program of China (2012CB518000, 2013CB531200, 2012CB944501) and the National Natural Science Foundation of China (81070674, 81070116, 3/22/002 and 81130073).
Author information
Authors and Affiliations
Contributions
R.S., W.P. and Y.Z. are equally contributing first authors. R.S. generated the initial idea and conducted key experiments. R.S., W.P., Y.Z., C.-M.C. and R.-P.X. designed the study, analysed the data and wrote the manuscript. C.-M.C. and R.-P.X. interpreted significance of the study. R.S., W.P., Y.Z., F. Lv, H.-K.W., J.G., Y.C., Y.P., Xin Z., L.J., M.Z., Pe.J., F. Liu and S.M. performed the experiments. Pi.J. helped in the generation of MG53 transgenic mice. Xiu.Z. provided the nonhuman primate tissues.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-19. (PDF 2423 kb)
Rights and permissions
About this article
Cite this article
Song, R., Peng, W., Zhang, Y. et al. Central role of E3 ubiquitin ligase MG53 in insulin resistance and metabolic disorders. Nature 494, 375–379 (2013). https://doi.org/10.1038/nature11834
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11834
This article is cited by
-
Cardiac-to-adipose axis in metabolic homeostasis and diseases: special instructions from the heart
Cell & Bioscience (2023)
-
Bibliometric and visual analysis of diabetes mellitus and pyroptosis from 2011 to 2022
European Journal of Medical Research (2023)
-
Targeting E3 ubiquitin ligases and their adaptors as a therapeutic strategy for metabolic diseases
Experimental & Molecular Medicine (2023)
-
Structural basis for TRIM72 oligomerization during membrane damage repair
Nature Communications (2023)
-
Targeting protein modifications in metabolic diseases: molecular mechanisms and targeted therapies
Signal Transduction and Targeted Therapy (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.