Abstract
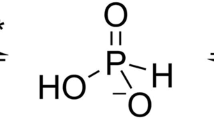
Arsenate and phosphate are abundant on Earth and have striking similarities: nearly identical pKa values1,2, similarly charged oxygen atoms, and thermochemical radii that differ by only 4% (ref. 3). Phosphate is indispensable and arsenate is toxic, but this extensive similarity raises the question whether arsenate may substitute for phosphate in certain niches4,5. However, whether it is used or excluded, discriminating phosphate from arsenate is a paramount challenge. Enzymes that utilize phosphate, for example, have the same binding mode and kinetic parameters as arsenate, and the latter’s presence therefore decouples metabolism6,7. Can proteins discriminate between these two anions, and how would they do so? In particular, cellular phosphate uptake systems face a challenge in arsenate-rich environments. Here we describe a molecular mechanism for this process. We examined the periplasmic phosphate-binding proteins (PBPs) of the ABC-type transport system that mediates phosphate uptake into bacterial cells, including two PBPs from the arsenate-rich Mono Lake Halomonas strain GFAJ-1. All PBPs tested are capable of discriminating phosphate over arsenate at least 500-fold. The exception is one of the PBPs of GFAJ-1 that shows roughly 4,500-fold discrimination and its gene is highly expressed under phosphate-limiting conditions. Sub-ångström-resolution structures of Pseudomonas fluorescens PBP with both arsenate and phosphate show a unique mode of binding that mediates discrimination. An extensive network of dipole–anion interactions8,9, and of repulsive interactions, results in the 4% larger arsenate distorting a unique low-barrier hydrogen bond. These features enable the phosphate transport system to bind phosphate selectively over arsenate (at least 103 excess) even in highly arsenate-rich environments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
Primary accessions
Protein Data Bank
Data deposits
Coordinates and structure factors for the arsenate-bound PfluDING structures at pH4.5 and 8.5 are deposited in the Protein Data Bank (accession codes 4F18 and 4F19); the phosphate-bound PfluDING coordinates and structures at pH4.5 and 8.5 (re-refinement) are deposited in the Protein Data Bank (accessioncodes 4F1U and 4F1V).
References
Kohn, R. A. & Dunlap, T. F. Calculation of the buffering capacity of bicarbonate in the rumen and in vitro. J. Anim. Sci. 76, 1702–1709 (1998)
Larsen, E. H. & Hansen, S. H. Separation of arsenic species by ion-pair and ion exchange high performance liquid chromatography. Microchim. Acta 109, 47–51 (1992)
Kish, M. M. & Viola, R. E. Oxyanion specificity of aspartate-β-semialdehyde dehydrogenase. Inorg. Chem. 38, 818–820 (1999)
Westheimer, F. H. Why nature chose phosphates. Science 235, 1173–1178 (1987)
Wolfe-Simon, F. et al. A bacterium that can grow by using arsenic instead of phosphorus. Science 332, 1163–1166 (2011)
Dixon, H. B. F. The biochemical action of arsonic acids especially as phosphate analogues. Adv. Inorg. Chem. 44, 191–227 (1997)
Tawfik, D. S. & Viola, R. E. Arsenate replacing phosphate: alternative life chemistries and ion promiscuity. Biochemistry 50, 1128–1134 (2011)
Luecke, H. & Quiocho, F. A. High specificity of a phosphate transport protein determined by hydrogen bonds. Nature 347, 402–406 (1990)
Quiocho, F. A. & Ledvina, P. S. Atomic structure and specificity of bacterial periplasmic receptors for active transport and chemotaxis: variation of common themes. Mol. Microbiol. 20, 17–25 (1996)
Willsky, G. R. & Malamy, M. H. Characterization of two genetically separable inorganic phosphate transport systems in Escherichia coli. J. Bacteriol. 144, 356–365 (1980)
Rao, N. N. & Torriani, A. Molecular aspects of phosphate transport in Escherichia coli. Mol. Microbiol. 4, 1083–1090 (1990)
Willsky, G. R. & Malamy, M. H. Effect of arsenate on inorganic phosphate transport in Escherichia coli. J. Bacteriol. 144, 366–374 (1980)
Quiocho, F. A. Atomic structures of periplasmic binding proteins and the high-affinity active transport systems in bacteria. Phil. Trans. R. Soc. Lond. B 326, 341–351 (1990)
Butt, A. & Rehman, A. Isolation of arsenite-oxidizing bacteria from industrial effluents and their potential use in wastewater treatment. World J. Microbiol. Biotechnol. 27, 2435–2441 (2011)
Reaves, M. L., Sinha, S., Rabinowitz, J. D., Kruglyak, L. & Redfield, R. J. Absence of detectable arsenate in DNA from arsenate-grown GFAJ-1 cells. Science 337, 470–473 (2012)
Erb, T. J., Kiefer, P., Hattendorf, B., Gunther, D. & Vorholt, J. A. GFAJ-1 is an arsenate-resistant, phosphate-dependent organism. Science 337, 467–470 (2012)
Kim, E.-H. & Rensing, C. Genome of Halomonas strain GFAJ-1, a blueprint for fame or business as usual. J. Bacteriol. 194, 1643–1645 (2012)
Ahn, S. et al. Structure–function relationships in a bacterial DING protein. FEBS Lett. 581, 3455–3460 (2007)
Ledvina, P. S., Yao, N., Choudhary, A. & Quiocho, F. A. Negative electrostatic surface potential of protein sites specific for anionic ligands. Proc. Natl Acad. Sci. USA 93, 6786–6791 (1996)
Tanabe, M. et al. Structures of OppA and PstS from Yersinia pestis indicate variability of interactions with transmembrane domains. Acta Crystallogr. D 63, 1185–1193 (2007)
Vyas, N. K., Vyas, M. N. & Quiocho, F. A. Crystal structure of M. tuberculosis ABC phosphate transport receptor: specificity and charge compensation dominated by ion–dipole interactions. Structure 11, 765–774 (2003)
Liebschner, D. et al. Elucidation of the phosphate binding mode of DING proteins revealed by subangstrom X-ray crystallography. J. Am. Chem. Soc. 131, 7879–7886 (2009)
Faehnle, C. R., Blanco, J. & Viola, R. E. Structural basis for discrimination between oxyanion substrates or inhibitors in aspartate-β-semialdehyde dehydrogenase. Acta Crystallogr. D 60, 2320–2324 (2004)
Kline, P. C. & Schramm, V. L. Purine nucleoside phosphorylase. Catalytic mechanism and transition-state analysis of the arsenolysis reaction. Biochemistry 32, 13212–13219 (1993)
Wang, Z., Luecke, H., Yao, N. & Quiocho, F. A. A low energy short hydrogen bond in very high resolution structures of protein receptor–phosphate complexes. Nature Struct. Biol. 4, 519–522 (1997)
Cleland, W. W. Low-barrier hydrogen bonds and enzymatic catalysis. Arch. Biochem. Biophys. 382, 1–5 (2000)
Steiner, T. & Saenger, W. Lengthening of the covalent O–H bond in O–H…O hydrogen bonds re-examined from low-temperature neutron diffraction data of organic compounds. Acta Crystallogr. B 50, 348–357 (1994)
Khersonsky, O. & Tawfik, D. S. Enzyme promiscuity: a mechanistic and evolutionary perspective. Annu. Rev. Biochem. 79, 471–505 (2010)
Li, A. J. & Nussinov, R. A set of van der Waals and coulombic radii of protein atoms for molecular and solvent-accessible surface calculation, packing evaluation, and docking. Proteins 32, 111–127 (1998)
Pflugrath, J. W. & Quiocho, F. A. Sulphate sequestered in the sulphate-binding protein of Salmonella typhimurium is bound solely by hydrogen bonds. Nature 314, 257–260 (1985)
Acknowledgements
We thank G. Gotthard for valuable inputs, C. Jelsch for help with the software Mopro, and A. Toth-Petroczy for help with the phylogenetic analysis. Financial support by the Israel Science Foundation is gratefully acknowledged. M.E. is a fellow supported by the Intra-European Fellowships Marie Curie program (grant No. 252836). D.S.T. is the Nella and Leon Benoziyo Professor of Biochemistry. T.J.E. was supported by an Eidgenössische Technische Hochschule fellowship.
Author information
Authors and Affiliations
Contributions
M.E. initiated the project, designed experiments, crystallized the proteins, collected and processed crystallographic data, performed discrimination assays, analysed the data and wrote the manuscript. A.W. cloned, purified and crystallized proteins, performed discrimination assays and analysed data. K.G.A. cloned and constructed the mutants, purified proteins and performed discrimination assays. T.J.E. and J.A.V. designed, performed and analysed reverse transcription PCR, as well as cellular experiments, and provided physiological data. E.C. purified proteins and collected diffraction data. D.S.T. initiated the project, designed experiments, analysed data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-13, Supplementary Methods, Supplementary Tables 1-18 and additional references. (PDF 3199 kb)
Rights and permissions
About this article
Cite this article
Elias, M., Wellner, A., Goldin-Azulay, K. et al. The molecular basis of phosphate discrimination in arsenate-rich environments. Nature 491, 134–137 (2012). https://doi.org/10.1038/nature11517
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11517
This article is cited by
-
Transcriptomics, proteomics, and metabolomics interventions prompt crop improvement against metal(loid) toxicity
Plant Cell Reports (2024)
-
Blooming of a microbial community in an Ediacaran extreme volcanic lake system
Scientific Reports (2023)
-
Neutron crystallography reveals mechanisms used by Pseudomonas aeruginosa for host-cell binding
Nature Communications (2022)
-
Mapping periplasmic binding protein oligosaccharide recognition with neutron crystallography
Scientific Reports (2022)
-
Are cysteine, glutathione and phytochelatins responses of Myriophyllum alterniflorum to copper and arsenic stress affected by trophic conditions?
BioMetals (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.