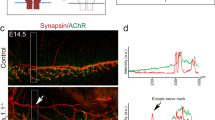
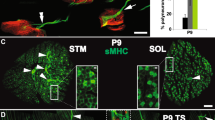
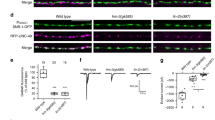
Abstract
Motor axons receive retrograde signals from skeletal muscle that are essential for the differentiation and stabilization of motor nerve terminals1. Identification of these retrograde signals has proved elusive, but their production by muscle depends on the receptor tyrosine kinase, MuSK (muscle, skeletal receptor tyrosine-protein kinase), and Lrp4 (low-density lipoprotein receptor (LDLR)-related protein 4), an LDLR family member that forms a complex with MuSK, binds neural agrin and stimulates MuSK kinase activity2,3,4,5. Here we show that Lrp4 also functions as a direct muscle-derived retrograde signal for early steps in presynaptic differentiation. We demonstrate that Lrp4 is necessary, independent of MuSK activation, for presynaptic differentiation in vivo, and we show that Lrp4 binds to motor axons and induces clustering of synaptic-vesicle and active-zone proteins. Thus, Lrp4 acts bidirectionally and coordinates synapse formation by binding agrin, activating MuSK and stimulating postsynaptic differentiation, and functioning in turn as a muscle-derived retrograde signal that is necessary and sufficient for presynaptic differentiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sanes, J. R. & Lichtman, J. W. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nature Rev. Neurosci. 2, 791–805 (2001)
DeChiara, T. M. et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell 85, 501–512 (1996)
Weatherbee, S. D., Anderson, K. V. & Niswander, L. A. LDL-receptor-related protein 4 is crucial for formation of the neuromuscular junction. Development 133, 4993–5000 (2006)
Kim, N. et al. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell 135, 334–342 (2008)
Zhang, B. et al. LRP4 serves as a coreceptor of agrin. Neuron 60, 285–297 (2008)
Arber, S., Burden, S. J. & Harris, A. J. Patterning of skeletal muscle. Curr. Opin. Neurobiol. 12, 100–103 (2002)
Yang, X. et al. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron 30, 399–410 (2001)
Yang, X., Li, W., Prescott, E. D., Burden, S. J. & Wang, J. C. DNA topoisomerase IIbeta and neural development. Science 287, 131–134 (2000)
Lin, W. et al. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature 410, 1057–1064 (2001)
Panzer, J. A. S. o. n. g. Y. & Balice-Gordon, R. J. In vivo imaging of preferential motor axon outgrowth to and synaptogenesis at prepatterned acetylcholine receptor clusters in embryonic zebrafish skeletal muscle. J. Neurosci. 26, 934–947 (2006)
Flanagan-Steet, H., Fox, M. A., Meyer, D. & Sanes, J. R. Neuromuscular synapses can form in vivo by incorporation of initially aneural postsynaptic specializations. Development 132, 4471–4481 (2005)
Burden, S. J. SnapShot: neuromuscular junction. Cell 144, 826.e1 (2011)
Kummer, T. T., Misgeld, T. & Sanes, J. R. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr. Opin. Neurobiol. 16, 74–82 (2006)
Zhang, W., Coldefy, A. S., Hubbard, S. R. & Burden, S. J. Agrin binds to the N-terminal region of Lrp4 and stimulates association between Lrp4 and the first Ig-like domain in MuSK. J. Biol. Chem. (2011)
Gautam, M. et al. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell 85, 525–535 (1996)
Lin, W. et al. Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism. Neuron 46, 569–579 (2005)
Misgeld, T., Kummer, T. T., Lichtman, J. W. & Sanes, J. R. Agrin promotes synaptic differentiation by counteracting an inhibitory effect of neurotransmitter. Proc. Natl Acad. Sci. USA 102, 11088–11093 (2005)
Kim, N. & Burden, S. J. MuSK controls where motor axons grow and form synapses. Nature Neurosci. 11, 19–27 (2008)
Gomez, A. M. &. B. u. r. d. e. n. S. J. The extracellular region of Lrp4 is sufficient to mediate neuromuscular synapse formation. Dev. Dynam. 240, 2626–2633 (2011)
Engel, A. G., Ohno, K. & Sine, S. M. Sleuthing molecular targets for neurological diseases at the neuromuscular junction. Nature Rev. Neurosci. 4, 339–352 (2003)
Higuchi, O., Hamuro, J., Motomura, M. & Yamanashi, Y. Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Ann. Neurol. 69, 418–422 (2011)
Drescher, U. The Eph family in the patterning of neural development. Curr. Biol. 7, R799–R807 (1997)
Bao, J., Wolpowitz, D., Role, L. W. & Talmage, D. A. Back signaling by the Nrg-1 intracellular domain. J. Cell Biol. 161, 1133–1141 (2003)
Fox, M. A. et al. Distinct target-derived signals organize formation, maturation, and maintenance of motor nerve terminals. Cell 129, 179–193 (2007)
Higuchi, O., Hamuro, J., Motomura, M. & Yamanashi, Y. Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Ann. Neurol. 69, 418–422 (2011)
Pun, S., Santos, A. F., Saxena, S., Xu, L. & Caroni, P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nature Neurosci. 9, 408–419 (2006)
Valdez, G. et al. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc. Natl Acad. Sci. USA 107, 14863–14868 (2010)
Hata, K., Polo-Parada, L. & Landmesser, L. T. Selective targeting of different neural cell adhesion molecule isoforms during motoneuron myotube synapse formation in culture and the switch from an immature to mature form of synaptic vesicle cycling. J. Neurosci. 27, 14481–14493 (2007)
Umemori, H., Linhoff, M. W., Ornitz, D. M. & Sanes, J. R. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell 118, 257–270 (2004)
Umemori, H. & Sanes, J. R. Signal regulatory proteins (SIRPS) are secreted presynaptic organizing molecules. J. Biol. Chem. 283, 34053–34061 (2008)
Acknowledgements
We thank L. Landmesser and K. Hata for helping us to establish a motor-neuron and muscle co-culture system, W. Zhang for constructs encoding Fc- and AP-tagged forms of Lrp4, T. Jessell and J. Dasen for HB9::GFP mice, J. Sanes for agrin-mutant mice, K. Anderson and L. Niswander for Lrp4-mutant mice, and D. Littman and R. Lehmann for comments on the manuscript. We are grateful to P. Lopez and M. J. Sunshine and personnel in the transgenic mouse core for their assistance. The flow cytometry and transgenic mouse cores are supported by a New York University Cancer Institute Center Support Grant (National Institutes of Health (NIH)/National Cancer Institute (NCI) 5 P30CA16087-31). This work was supported with funds from the National Institutes of Health (NS36193 to S.J.B.), the Skirball Institute and postdoctoral training support to N.Y. from New York State Stem Cell Science (NYSTEM).
Author information
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-9. (PDF 7501 kb)
Rights and permissions
About this article
Cite this article
Yumoto, N., Kim, N. & Burden, S. Lrp4 is a retrograde signal for presynaptic differentiation at neuromuscular synapses. Nature 489, 438–442 (2012). https://doi.org/10.1038/nature11348
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11348
This article is cited by
-
Development and characterization of agonistic antibodies targeting the Ig-like 1 domain of MuSK
Scientific Reports (2023)
-
Intrafusal-fiber LRP4 for muscle spindle formation and maintenance in adult and aged animals
Nature Communications (2023)
-
CRABP1-CaMKII-Agrn regulates the maintenance of neuromuscular junction in spinal motor neuron
Cell Death & Differentiation (2022)
-
Impaired signaling for neuromuscular synaptic maintenance is a feature of Motor Neuron Disease
Acta Neuropathologica Communications (2022)
-
Synapse-specific Lrp4 mRNA enrichment requires Lrp4/MuSK signaling, muscle activity and Wnt non-canonical pathway
Cell & Bioscience (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.