Abstract
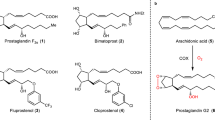
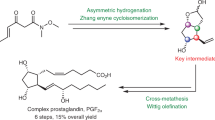
Prostaglandins are hormone-like chemical messengers that regulate a broad range of physiological activities, including blood circulation, digestion and reproduction1,2. Their biological activities and their complex molecular architectures have made prostaglandins popular targets for synthetic organic chemists for over 40 years3,4. Prostaglandin analogues are widely used as pharmaceuticals and some, such as latanoprost, which is used to treat glaucoma5,6, have become billion-dollar drugs. Previously reported syntheses of these compounds are quite lengthy, and every chemical step costs time and energy, generates waste and is accompanied by material losses. Using a new bond disconnection, here we report a concise synthesis of the most complex prostaglandin, PGF2α, with high levels of control of relative and absolute stereochemistry, and fewer steps. The key step is an aldol cascade reaction of succinaldehyde using proline organocatalysis to create a bicyclic enal in one step and an enantiomeric excess of 98%. This intermediate bicyclic enal is fully primed with the appropriate functionality for attachment of the remaining groups7. Access to this bicyclic enal will not only render existing prostaglandin-based drugs more affordable, but will also facilitate the rapid exploration of related chemical structures around the ubiquitous five-membered ring motif, such as potentially therapeutic prostaglandin analogues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Funk, C. D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294, 1871–1875 (2001)
Bindra, J. S. & Bindra, R. Prostaglandin Synthesis (Academic Press, 1977)
Bindra, J. S. in The Total Synthesis of Natural Products (ed. ApSimon, J. ) Vol. 4 353–449 (Wiley, 1981)
Das, S., Chandrasekhar, S., Yadav, J. S. & Gree, R. Recent developments in the synthesis of prostaglandins and analogues. Chem. Rev. 107, 3286–3337 (2007)
Collins, P. W. & Djuric, S. W. Synthesis of therapeutically useful prostaglandin and prostacyclin analogs. Chem. Rev. 93, 1533–1564 (1993)
Nair, S. K. & Henegar, K. E. in Modern Drug Synthesis (eds Li, J. J. & Johnson, D. S. ) Ch. 21 329–338 (Wiley, 2010)
Ungrin, M. D. et al. Key structural features of prostaglandin E2 and prostanoid analogs involved in binding and activation of the human EP1 prostanoid receptor. Mol. Pharmacol. 59, 1446–1456 (2001)
Stix, G. Better ways to target pain. Sci. Am. 68–71, http://www.scientificamerican.com/article.cfm?id=better-ways-to-target-pain (16 December 2006)
Von Euler, U. S. Information on the pharmacological effect of natural secretions and extracts from male accessory sexual glands. Arch. Exp. Pathol. Pharm. 175, 78–84 (1934)
Flower, R. J. Prostaglandins, bioassay and inflammation. Br. J. Pharmacol. 147, S182–S192 (2006)
Woodward, R. B. et al. Novel synthesis of prostaglandin F2α . J. Am. Chem. Soc. 95, 6853–6855 (1973)
Corey, E. J. & Cheng, X.-M. The Logic of Chemical Synthesis (Wiley, 1995)
Stork, G., Sher, P. M. & Chen, H. L. Radical cyclization-trapping in the synthesis of natural products. A simple, stereocontrolled route to prostaglandin F2α . J. Am. Chem. Soc. 108, 6384–6385 (1986)
Suzuki, M., Yanagisawa, A. & Noyori, R. Prostaglandin synthesis. 16. The three-component coupling synthesis of prostaglandins. J. Am. Chem. Soc. 110, 4718–4726 (1988)
Danishefsky, S. J., Paz Cabal, M. & Chow, K. Novel stereospecific silyl group transfer reactions: practical routes to the prostaglandins. J. Am. Chem. Soc. 111, 3456–3457 (1989)
Resul, B. et al. Phenyl-substituted prostaglandins: potent and selective antiglaucoma agents. J. Med. Chem. 36, 243–248 (1993)
Pfizer . reports fourth-quarter and full-year 2010 results; provides 2011 financial guidance and updates 2012 financial targets. http://www.pfizer.com/files/investors/presentations/q4performance_020111.pdf (2011)
Corey, E. J., Weinshenker, N. M., Schaaf, T. K. & Huber, W. Stereo-controlled synthesis of dl-prostaglandins F2α and E2 . J. Am. Chem. Soc. 91, 5675–5677 (1969)
Howard, C. C. et al. Total synthesis of prostaglandin-F2α involving stereocontrolled and photo-induced reactions of bicyclo[3.2.0]heptanones. J. Chem. Soc. Perkin Trans. I 852–857 (1980)
Corey, E. J., Nicolaou, K. C. & Beames, D. J. A short synthetic route to prostaglandins utilizing position-selective epoxide opening by the vinyl Gilman reagent. Tetrahedr. Lett. 15, 2439–2440 (1974)
Mukherjee, S., Yang, J. W., Hoffmann, S. & List, B. Asymmetric enamine catalysis. Chem. Rev. 107, 5471–5569 (2007)
List, B., Lerner, R. A. & Barbas, C. F. Proline-catalyzed direct asymmetric aldol reactions. J. Am. Chem. Soc. 122, 2395–2396 (2000)
Northrup, A. B. & MacMillan, D. W. C. The first direct and enantioselective cross-aldol reaction of aldehydes. J. Am. Chem. Soc. 124, 6798–6799 (2002)
Corey, E. J. & Ravindranathan, T. A simple route to a key intermediate for the synthesis of 11-desoxyprostaglandins. Tetrahedr. Lett. 12, 4753–4755 (1971)
Corey, E. J. et al. Stereospecific total synthesis of gibberellic acid. A key tricyclic intermediate. J. Am. Chem. Soc. 100, 8031–8034 (1978)
Bahmanyar, S., Houk, K. N., Martin, H. J. & List, B. Quantum mechanical predictions of the stereoselectivities of proline-catalyzed asymmetric intermolecular aldol reactions. J. Am. Chem. Soc. 125, 2475–2479 (2003)
Gourlay, B. S., Molesworth, P. P., Ryan, J. H. & Smith, J. A. A new and high yielding synthesis of unstable pyrroles via a modified Clauson-Kaas reaction. Tetrahedr. Lett. 47, 799–801 (2006)
Lipshutz, B. H., Kozlowski, J. A., Parker, D. A., Nguyen, S. L. & McCarthy, K. E. More highly mixed, higher order cyanocuprates “RT(2-thieny)Cu(CN)Li2”. Efficient reagents which promote selective ligand transfer. J. Organomet. Chem. 285, 437–447 (1985)
de los Angeles Rey, M. et al. New synthetic strategies to vitamin D analogues modified at the side chain and D ring. Synthesis of 1α,25-dihydroxy-16-ene-vitamin D3 and C-20 analogues. J. Org. Chem. 64, 3196–3206 (1999)
Sheddan, N. A. & Mulzer, J. Cross metathesis as a general strategy for the synthesis of prostacyclin and prostaglandin analogues. Org. Lett. 8, 3101–3104 (2006)
Acknowledgements
We thank EPSRC and the European Research Council (FP7/2007-2013, ERC grant no. 246785) for financial support. V.K.A. thanks the Royal Society for a Wolfson Research Merit Award and EPSRC for a Senior Research Fellowship.
Author information
Authors and Affiliations
Contributions
G.C. and W.E. were involved in the discovery and subsequent development of the aldol reaction and G.C. applied it to PGF2α. V.K.A. conceived and directed the investigations and composed the manuscript with revisions provided by G.C. and W.E.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data, Supplementary Figures and Graphs, Supplementary Tables 1-10 and Supplementary References. (PDF 3917 kb)
Rights and permissions
About this article
Cite this article
Coulthard, G., Erb, W. & Aggarwal, V. Stereocontrolled organocatalytic synthesis of prostaglandin PGF2α in seven steps. Nature 489, 278–281 (2012). https://doi.org/10.1038/nature11411
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11411
This article is cited by
-
Concise, scalable and enantioselective total synthesis of prostaglandins
Nature Chemistry (2021)
-
Mass spectrometry and multivariate analysis to classify cervical intraepithelial neoplasia from blood plasma: an untargeted lipidomic study
Scientific Reports (2018)
-
Decarboxylative alkenylation
Nature (2017)
-
Synthesis of (1H-pyrrol-1-yl)anthracene-9,10-diones
Chemistry of Heterocyclic Compounds (2016)
-
15d-Prostaglandin J2 induced reactive oxygen species-mediated apoptosis during experimental visceral leishmaniasis
Journal of Molecular Medicine (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.