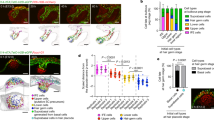
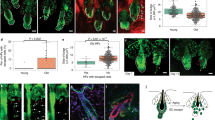
Abstract
Tissue development and regeneration depend on cell–cell interactions and signals that target stem cells and their immediate progeny1. However, the cellular behaviours that lead to a properly regenerated tissue are not well understood. Using a new, non-invasive, intravital two-photon imaging approach we study physiological hair-follicle regeneration over time in live mice. By these means we have monitored the behaviour of epithelial stem cells and their progeny2,3,4 during physiological hair regeneration and addressed how the mesenchyme5 influences their behaviour. Consistent with earlier studies6, stem cells are quiescent during the initial stages of hair regeneration, whereas the progeny are more actively dividing. Moreover, stem cell progeny divisions are spatially organized within follicles. In addition to cell divisions, coordinated cell movements of the progeny allow the rapid expansion of the hair follicle. Finally, we show the requirement of the mesenchyme for hair regeneration through targeted cell ablation and long-term tracking of live hair follicles. Thus, we have established an in vivo approach that has led to the direct observation of cellular mechanisms of growth regulation within the hair follicle and that has enabled us to precisely investigate functional requirements of hair-follicle components during the process of physiological regeneration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Morrison, S. J. & Spradling, A. C. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611 (2008)
Cotsarelis, G., Sun, T. T. & Lavker, R. M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61, 1329–1337 (1990)
Tumbar, T. et al. Defining the epithelial stem cell niche in skin. Science 303, 359–363 (2004)
Oshima, H., Rochat, A., Kedzia, C., Kobayashi, K. & Barrandon, Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 104, 233–245 (2001)
Jahoda, C. A., Horne, K. A. & Oliver, R. F. Induction of hair growth by implantation of cultured dermal papilla cells. Nature 311, 560–562 (1984)
Greco, V. et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 4, 155–169 (2009)
Xie, Y. et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature 457, 97–101 (2009)
Lo Celso, C. et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature 457, 92–96 (2009)
Uchugonova, A., Hoffman, R. M., Weinigel, M. & Koenig, K. Watching stem cells in the skin of living mice noninvasively. Cell Cycle 10, 2017–2020 (2011)
Ra, H. et al. In vivo imaging of human and mouse skin with a handheld dual-axis confocal fluorescence microscope. J. Invest. Dermatol. 131, 1061–1066 (2011)
Watt, F. M. & Jensen, K. B. Epidermal stem cell diversity and quiescence. EMBO Mol. Med. 1, 260–267 (2009)
Fuchs, E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell 137, 811–819 (2009)
Greco, V. & Guo, S. Compartmentalized organization: a common and required feature of stem cell niches? Development 137, 1586–1594 (2010)
Waghmare, S. K. et al. Quantitative proliferation dynamics and random chromosome segregation of hair follicle stem cells. EMBO J. 27, 1309–1320 (2008)
Müller-Röver, S. et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Invest. Dermatol. 117, 3–15 (2001)
Wallingford, J. B., Fraser, S. E. & Harland, R. M. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev. Cell 2, 695–706 (2002)
Zhang, Y. V., Cheong, J., Ciapurin, N., McDermitt, D. J. & Tumbar, T. Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell 5, 267–278 (2009)
Petersson, M. et al. TCF/Lef1 activity controls establishment of diverse stem and progenitor cell compartments in mouse epidermis. EMBO J. 30, 3004–3018 (2011)
Rubin, L. & Saunders, J. W., Jr Ectodermal–mesodermal interactions in the growth of limb buds in the chick embryo: constancy and temporal limits of the ectodermal induction. Dev. Biol. 28, 94–112 (1972)
Plikus, M. V. et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 451, 340–344 (2008)
Rendl, M., Lewis, L. & Fuchs, E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 3, e331 (2005)
Eiraku, M. et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56 (2011)
Acknowledgements
We are grateful to A. Horwich, V. Reinke and A. Giraldez for critical discussion of the work. We thank S. King for feedback on the manuscript, and E. Fuchs for the K14H2BGFP, Lef1RFP and pTREH2BGFP mice and A. Glick for the K5tta mice. P.R. is supported by the James Hudson Brown – Alexander Brown Coxe postdoctoral fellowship. G.Z. is a New York Stem Cell Foundation–Druckenmiller Fellow. E.D. is supported by the Cell and Molecular Biology Training Grant of the National Institute of General Medical Sciences, US National Institutes of Health, Public Health Service. This work was supported in part by the American Skin Association, the American Cancer Society and the Yale Rheumatologic Disease Research Core Center grant NIH P30AR053495.
Author information
Authors and Affiliations
Contributions
Author Contributions P.R. and V.G. designed experiments and wrote the manuscript, and P.R. performed the experiments and analysed the data. E.D. performed two-photon laser-scanning timelapses. G.Z. performed immunostainings. I.S. set up the mouse colonies and staining protocols. D.G. and A.H. assisted on initial intravital imaging set-up. D.G. performed data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-9 and Legends for Supplementary Movies 1-9. (PDF 8970 kb)
Supplementary Movie 1
This movie shows serial optical sections of in vivo mouse skin imaging (see Supplementary information file for full legend). (MOV 2393 kb)
Supplementary Movie 2
This movie shows cell divisions visualized in real-time in the hair follicle stem cell progeny compartment (see Supplementary information file for full legend). (MOV 6223 kb)
Supplementary Movie 3
This movie shows cell divisions visualized in real-time in the hair follicle stem cell progeny compartment (see Supplementary information file for full legend). (MOV 1679 kb)
Supplementary Movie 4
This movie shows cell divisions visualized in real-time in the hair follicle stem cell compartment (see Supplementary information file for full legend). (MOV 4920 kb)
Supplementary Movie 5
This movie shows rapid downward extension of the progeny compartment of the hair follicle during growth (see Supplementary information file for full legend). (MOV 1479 kb)
Supplementary Movie 6
This movie shows rapid downward extension of the progeny compartment of the hair follicle during growth (see Supplementary information file for full legend). (MOV 488 kb)
Supplementary Movie 7
This movie shows re-organization of the progeny compartment of the hair follicle around the mesenchyme during growth (see Supplementary information file for full legend). (MOV 3636 kb)
Supplementary Movie 8
This movie shows downward migration of nuclei in the hair follicle (see Supplementary information file for full legend). (MOV 1356 kb)
Supplementary Movie 9
This movie shows upward migration of nuclei in the hair follicle bulge (see Supplementary information file for full legend). (MOV 1376 kb)
Rights and permissions
About this article
Cite this article
Rompolas, P., Deschene, E., Zito, G. et al. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature 487, 496–499 (2012). https://doi.org/10.1038/nature11218
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11218
This article is cited by
-
Local and systemic mechanisms that control the hair follicle stem cell niche
Nature Reviews Molecular Cell Biology (2024)
-
Lrig1-expressing epidermal progenitors require SCD1 to maintain the dermal papilla niche
Scientific Reports (2023)
-
Hdac1 and Hdac2 regulate the quiescent state and survival of hair-follicle mesenchymal niche
Nature Communications (2023)
-
Dedifferentiation maintains melanocyte stem cells in a dynamic niche
Nature (2023)
-
Regulation and dysregulation of hair regeneration: aiming for clinical application
Cell Regeneration (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.