Abstract
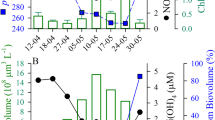
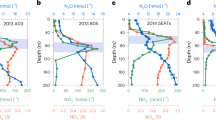
Fertilization of the ocean by adding iron compounds has induced diatom-dominated phytoplankton blooms accompanied by considerable carbon dioxide drawdown in the ocean surface layer. However, because the fate of bloom biomass could not be adequately resolved in these experiments, the timescales of carbon sequestration from the atmosphere are uncertain. Here we report the results of a five-week experiment carried out in the closed core of a vertically coherent, mesoscale eddy of the Antarctic Circumpolar Current, during which we tracked sinking particles from the surface to the deep-sea floor. A large diatom bloom peaked in the fourth week after fertilization. This was followed by mass mortality of several diatom species that formed rapidly sinking, mucilaginous aggregates of entangled cells and chains. Taken together, multiple lines of evidence—although each with important uncertainties—lead us to conclude that at least half the bloom biomass sank far below a depth of 1,000 metres and that a substantial portion is likely to have reached the sea floor. Thus, iron-fertilized diatom blooms may sequester carbon for timescales of centuries in ocean bottom water and for longer in the sediments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sigman, D. M. Hain, M. P. & Haug, G. H. The polar ocean and glacial cycles in atmospheric CO2 concentration. Nature 466, 47–55 (2010)
Anderson, R. F. et al. Wind-driven upwelling in the Southern Ocean and the deglacial rise in atmospheric CO2 . Science 323, 1443–1448 (2009)
Martin, J. H. Glacial-interglacial CO2 changes: the iron hypothesis. Paleoceanography 5, 1–13 (1990)
Coale, K. H. et al. Southern Ocean iron enrichment experiment: carbon cycling in high- and low-Si waters. Science 304, 408–414 (2004)
Boyd, P. et al. Mesoscale iron-enrichment experiments 1993–2005: synthesis and future directions. Science 315, 612–617 (2007)
Cassar, N. et al. The Southern Ocean biological response to aeolian iron deposition. Science 317, 1067–1070 (2007)
Hamme, R. C. et al. Volcanic ash fuels anomalous plankton bloom in subarctic northeast Pacific. Geophys. Res. Lett. 37, L19604 (2010)
Lampitt, R. S. et al. Material supply to the abyssal seafloor in the Northeast Atlantic. Prog. Oceanogr. 50, 27–63 (2001)
Abelmann, A., Gersonde, R., Cortese, G., Kuhn, G. & Smetacek, V. Extensive phytoplankton blooms in the Atlantic sector of the glacial Southern Ocean. Paleoceanography 21, PA1013 (2006)
Kohfeld, K. E., Le Quéré, C., Harrison, S. P. & Anderson, R. F. Role of marine biology in glacial-interglacial CO2 cycles. Science 308, 74–78 (2005)
The Royal Society . Geoengineering the Climate: Science, Governance and Uncertainty. RS policy document 10/09 (The Royal Society, 2009)
Chelton, D. B., Schlax, M. G., Samelson, R. M. & de Szoeke, R. A. Global observations of large oceanic eddies. Geophys. Res. Lett. 34, L15606 (2007)
d'Ovidio, F., Isern-Fontanet, J., Lopez, C., Hernandez-Garcia, E. & Garcia-Ladona, E. Comparison between Eulerian diagnostics and finite-size Lyapunov exponents computed from altimetry in the Algerian basin. Deep Sea Res. Part I Oceanogr. Res. Pap. 56, 15–31 (2009)
Hibbert, A., Leach, H., Strass, V. & Cisewski, B. Mixing in cyclonic eddies in the Antarctic Circumpolar Current. J. Mar. Res. 67, 1–23 (2009)
Cisewski, B., Strass, V. H., Losch, M. & Prandke, H. Mixed layer analysis of a mesoscale eddy in the Antarctic Polar Front Zone. J. Geophys. Res. 113, C05017 (2008)
Jacquet, S. H. M., Savoye, N., Dehairs, F., Strass, V. H. & Cardinal, D. D. Mesopelagic carbon remineralization during the European Iron Fertilization Experiment. Glob. Biogeochem. Cycles 22, GB1023 (2008)
Paytan, A. & McLaughlin, K. The oceanic phosphorus cycle. Chem. Rev. 107, 563–576 (2007)
Bishop, J. K. B., Wood, T. J., Davis, R. E. & Sherman, J. T. Robotic observations of enhanced carbon biomass and export at 55° S during SOFeX. Science 304, 417–420 (2004)
Jackson, G. A. A model of the formation of marine algal flocs by physical coagulation processes. Deep-Sea Res. 37, 1197–1211 (1990)
Riebesell, U. & Wolf-Gladrow, D. A. The relationship between physical aggregation of phytoplankton and particle flux: a numerical model. Deep-Sea Res. A 39, 1085–1102 (1992)
de Baar, H. J. W. et al. Synthesis of iron fertilization experiments: from the iron age in the age of enlightenment. J. Geophys. Res. 110, C09S16 (2005)
Blain, S. et al. Effect of natural iron fertilization on carbon sequestration in the Southern Ocean. Nature 446, 1070–1074 (2007)
Pollard, R. et al. Southern Ocean deep-water carbon export enhanced by natural iron fertilization. Nature 457, 577–580 (2009)
Beaulieu, S. E. in Oceanography and Marine Biology. An Annual Review (eds Gibson, R. N., Barnes, M. & Atkinson, R. J. ) 171–232 (Taylor & Francis, 2002)
Acknowledgements
We thank C. Balt, K. Loquay, S. Mkatshwa, H. Prandke, H. Rohr, M. Thomas and I. Vöge for help on board. We are also grateful to U. Struck for POC and PON analyses. The altimeter products were produced by Ssalto/Duacs and distributed by Aviso with support from Cnes. We thank the captain and crew of RV Polarstern (cruise ANT XXI/3) for support throughout the cruise.
Author information
Authors and Affiliations
Contributions
V.S. and C.K. wrote the manuscript. V.S. directed the experiment and C.K. carried out the budget calculations. V.H.S., P.A., M.M. and D.W.-G. contributed to the preparation of the manuscript. V.H.S., B.C., H.L. and M.L. contributed physical data on mixed-layer depth dynamics, eddy coherence, patch movement and transmissometer data. N.S. provided thorium data. A.W. provided nutrient data. P.A. and J.H. provided phytoplankton and BSi data. F.D. carried out the Lagrangian analysis based on delayed-time altimetry. J.M.A. and G.J.H. provided bacterial data. C.N. and R.B. provided inorganic carbon data. G.M.B., C.K. and M.M.M. provided POC and PON data. P.C. provided the iron data. S.G. and A.T. provided DOM data. I.P. and L.J.H. performed the 14C primary production measurements and provided high-pressure liquid chromatography data. R.R. provided data on photochemical efficiency (Fv/Fm). C.K., M.M.S. and A.T. provided Chl data. U.B., E.S., O.S. and J.S. provided data on the eddy core from a subsequent cruise and satellite Chl images.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information 1
This file contains Supplementary Text and Data 1-5 (see contents). Each section also includes Supplementary Figures, Supplementary Tables and additional references. (PDF 14229 kb)
Supplementary Information 2
This file contains Supplementary Methods, additional references, Supplementary Figures 1-7 and Supplementary Tables 1-3. This file was replaced on 20 July 2012, as the figures that appeared in the original file were incorrect. (PDF 6258 kb)
Rights and permissions
About this article
Cite this article
Smetacek, V., Klaas, C., Strass, V. et al. Deep carbon export from a Southern Ocean iron-fertilized diatom bloom. Nature 487, 313–319 (2012). https://doi.org/10.1038/nature11229
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11229
This article is cited by
-
Wind-driven upwelling of iron sustains dense blooms and food webs in the eastern Weddell Gyre
Nature Communications (2023)
-
Potential use of engineered nanoparticles in ocean fertilization for large-scale atmospheric carbon dioxide removal
Nature Nanotechnology (2022)
-
Southern Ocean biogenic blooms freezing-in Oligocene colder climates
Nature Communications (2022)
-
Stable iron isotopic composition of atmospheric aerosols: An overview
npj Climate and Atmospheric Science (2022)
-
Using engineered nanoparticles to increase carbon dioxide storage in the ocean
Nature Nanotechnology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.