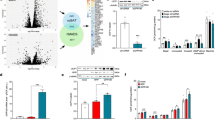
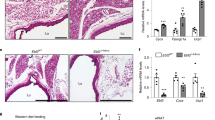
Abstract
Although feast and famine cycles illustrate that remodelling of adipose tissue in response to fluctuations in nutrient availability is essential for maintaining metabolic homeostasis, the underlying mechanisms remain poorly understood1,2. Here we identify fibroblast growth factor 1 (FGF1) as a critical transducer in this process in mice, and link its regulation to the nuclear receptor PPARγ (peroxisome proliferator activated receptor γ), which is the adipocyte master regulator and the target of the thiazolidinedione class of insulin sensitizing drugs3,4,5. FGF1 is the prototype of the 22-member FGF family of proteins and has been implicated in a range of physiological processes, including development, wound healing and cardiovascular changes6. Surprisingly, FGF1 knockout mice display no significant phenotype under standard laboratory conditions7,8,9. We show that FGF1 is highly induced in adipose tissue in response to a high-fat diet and that mice lacking FGF1 develop an aggressive diabetic phenotype coupled to aberrant adipose expansion when challenged with a high-fat diet. Further analysis of adipose depots in FGF1-deficient mice revealed multiple histopathologies in the vasculature network, an accentuated inflammatory response, aberrant adipocyte size distribution and ectopic expression of pancreatic lipases. On withdrawal of the high-fat diet, this inflamed adipose tissue fails to properly resolve, resulting in extensive fat necrosis. In terms of mechanisms, we show that adipose induction of FGF1 in the fed state is regulated by PPARγ acting through an evolutionarily conserved promoter proximal PPAR response element within the FGF1 gene. The discovery of a phenotype for the FGF1 knockout mouse establishes the PPARγ–FGF1 axis as critical for maintaining metabolic homeostasis and insulin sensitization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lee, M. J., Wu, Y. & Fried, S. K. Adipose tissue remodeling in pathophysiology of obesity. Curr. Opin. Clin. Nutr. Metab. Care 13, 371–376 (2010)
Sun, K., Kusminski, C. M. & Scherer, P. E. Adipose tissue remodeling and obesity. J. Clin. Invest. 121, 2094–2101 (2011)
Forman, B. M. et al. 15-Deoxy-Δ12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell 83, 803–812 (1995)
Barak, Y. et al. PPARγ is required for placental, cardiac, and adipose tissue development. Mol. Cell 4, 585–595 (1999)
Tontonoz, P. & Spiegelman, B. M. Fat and beyond: the diverse biology of PPARγ. Annu. Rev. Biochem. 77, 289–312 (2008)
Itoh, N. & Ornitz, D. M. Functional evolutionary history of the mouse Fgf gene family. Dev. Dyn. 237, 18–27 (2008)
Miller, D. L., Ortega, S., Bashayan, O., Basch, R. & Basilico, C. Compensation by fibroblast growth factor 1 (FGF1) does not account for the mild phenotypic defects observed in FGF2 null mice. Mol. Cell. Biol. 20, 2260–2268 (2000)
Beenken, A. & Mohammadi, M. The FGF family: biology, pathophysiology and therapy. Nature Rev. Drug Discov. 8, 235–253 (2009)
Itoh, N. & Ornitz, D. M. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J. Biochem. 149, 121–130 (2011)
Myers, R. L., Payson, R. A., Chotani, M. A., Deaven, L. L. & Chiu, I. M. Gene structure and differential expression of acidic fibroblast growth factor mRNA: identification and distribution of four different transcripts. Oncogene 8, 341–349 (1993)
Kanda, H. et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 116, 1494–1505 (2006)
Kamei, N. et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J. Biol. Chem. 281, 26602–26614 (2006)
Gesta, S., Tseng, Y. H. & Kahn, C. R. Developmental origin of fat: tracking obesity to its source. Cell 131, 242–256 (2007)
Schmitz-Moormann, P., von Wedel, R., Agricola, B. & Himmelmann, G. W. Studies of lipase-induced fat necrosis in rats. Pathol. Res. Pract. 163, 93–108 (1978)
Lee, P. C., Nakashima, Y., Appert, H. E. & Howard, J. M. Lipase and colipase in canine pancreatic juice as etiologic factors in fat necrosis. Surg. Gynecol. Obstet. 148, 39–44 (1979)
Chua, F. & Laurent, G. J. Neutrophil elastase: mediator of extracellular matrix destruction and accumulation. Proc. Am. Thorac. Soc. 3, 424–427 (2006)
Barish, G. D., Narkar, V. A. & Evans, R. M. PPARδ: a dagger in the heart of the metabolic syndrome. J. Clin. Invest. 116, 590–597 (2006)
Sugii, S. et al. PPARγ activation in adipocytes is sufficient for systemic insulin sensitization. Proc. Natl Acad. Sci. USA 106, 22504–22509 (2009)
Lefterova, M. I. et al. Cell-specific determinants of peroxisome proliferator-activated receptor γ function in adipocytes and macrophages. Mol. Cell. Biol. 30, 2078–2089 (2010)
He, W. et al. Adipose-specific peroxisome proliferator-activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. Proc. Natl Acad. Sci. USA 100, 15712–15717 (2003)
Hutley, L. et al. Fibroblast growth factor 1: a key regulator of human adipogenesis. Diabetes 53, 3097–3106 (2004)
Hutley, L. J. et al. A putative role for endogenous FGF-2 in FGF-1 mediated differentiation of human preadipocytes. Mol. Cell. Endocrinol. 339, 165–171 (2011)
Fon Tacer, K. et al. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol. Endocrinol. 24, 2050–2064 (2010)
Moore, D. D. Physiology. Sister act. Science 316, 1436–1438 (2007)
Kliewer, S. A. & Mangelsdorf, D. J. Fibroblast growth factor 21: from pharmacology to physiology. Am. J. Clin. Nutr. 91, 254S–257S (2010)
Kharitonenkov, A. FGFs and metabolism. Curr. Opin. Pharmacol. 9, 805–810 (2009)
Fang, S. et al. Corepressor SMRT promotes oxidative phosphorylation in adipose tissue and protects against diet-induced obesity and insulin resistance. Proc. Natl Acad. Sci. USA 108, 3412–3417 (2011)
Hevener, A. L. et al. Muscle-specific Pparg deletion causes insulin resistance. Nature Med. 9, 1491–1497 (2003)
Nofsinger, R. R. et al. SMRT repression of nuclear receptors controls the adipogenic set point and metabolic homeostasis. Proc. Natl Acad. Sci. USA 105, 20021–20026 (2008)
Barish, G. D. et al. Bcl-6 and NF-κB cistromes mediate opposing regulation of the innate immune response. Genes Dev. 24, 2760–2765 (2010)
Springer, M. L., Ip, T. K. & Blau, H. M. Angiogenesis monitored by perfusion with a space-filling microbead suspension. Mol. Ther. 1, 82–87 (2000)
Acknowledgements
We thank J. Alvarez, S. Kaufman, N. H. Uhlenhaut, M. Hassan and E. Williams for technical assistance, and L. Ong and S. Ganley for administrative assistance. R.M.E. is an Investigator of the Howard Hughes Medical Institute at the Salk Institute and March of Dimes Chair in Molecular and Developmental Biology. This work was supported by National Institutes of Health grants (DK062434, DK057978, DK090962, DK063491 and HL105278), the Helmsley Charitable Trust, and the Howard Hughes Medical Institute. J.W.J. is supported by the Human Frontier Science Program (HFSP), the Netherlands Organization for Scientific Research (NWO) and an EU Marie Curie Reintegration grant (IRG-277169). M.A. is supported by an F32 Ruth L. Kirschstein National Research Service Award (NIDDK).
Author information
Authors and Affiliations
Contributions
J.W.J, J.M.S, M.D. and R.M.E. designed and supervised the research. J.W.J., J.M.S., A.R.A., M.A., P.L., M.H., J.W., H.J., Y.-Q.Y. and C.T.P. performed research. R.R.H. provided samples and analysed results. J.W.J., J.M.S., R.T.Y., J.M.O., M.D. and R.M.E. analysed data. J.W.J, J.M.S., A.R.A., M.A., M.D. and R.M.E. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-8 and Supplementary Tables 1-3. (PDF 14267 kb)
Rights and permissions
About this article
Cite this article
Jonker, J., Suh, J., Atkins, A. et al. A PPARγ–FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature 485, 391–394 (2012). https://doi.org/10.1038/nature10998
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10998
This article is cited by
-
Impact of polygenic score for BMI on weight loss effectiveness and genome-wide association analysis
International Journal of Obesity (2024)
-
Blockage of PPARγ T166 phosphorylation enhances the inducibility of beige adipocytes and improves metabolic dysfunctions
Cell Death & Differentiation (2023)
-
Silencing of hypothalamic FGF11 prevents diet-induced obesity
Molecular Brain (2022)
-
The landscape of the long non-coding RNAs and circular RNAs of the abdominal fat tissues in the chicken lines divergently selected for fatness
BMC Genomics (2022)
-
Metabolic Messengers: fibroblast growth factor 1
Nature Metabolism (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.