Abstract
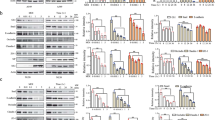
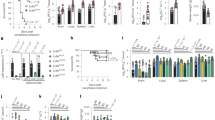
Measles virus is an aerosol-transmitted virus that affects more than 10 million children each year and accounts for approximately 120,000 deaths1,2. Although it was long believed to replicate in the respiratory epithelium before disseminating, it was recently shown to infect initially macrophages and dendritic cells of the airways using signalling lymphocytic activation molecule family member 1 (SLAMF1; also called CD150) as a receptor3,4,5,6. These cells then cross the respiratory epithelium and transport the infection to lymphatic organs where measles virus replicates vigorously7. How and where the virus crosses back into the airways has remained unknown. On the basis of functional analyses of surface proteins preferentially expressed on virus-permissive human epithelial cell lines, here we identify nectin-4 (ref. 8; also called poliovirus-receptor-like-4 (PVRL4)) as a candidate host exit receptor. This adherens junction protein of the immunoglobulin superfamily interacts with the viral attachment protein with high affinity through its membrane-distal domain. Nectin-4 sustains measles virus entry and non-cytopathic lateral spread in well-differentiated primary human airway epithelial sheets infected basolaterally. It is downregulated in infected epithelial cells, including those of macaque tracheae. Although other viruses use receptors to enter hosts or transit through their epithelial barriers, we suggest that measles virus targets nectin-4 to emerge in the airways. Nectin-4 is a cellular marker of several types of cancer9,10,11, which has implications for ongoing measles-virus-based clinical trials of oncolysis12.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
News Feature. Vaccines: the case of measles. Nature 473, 434–435 (2011)
Chen, S. Y. et al. Health care-associated measles outbreak in the united states after an importation: challenges and economic impact. J. Infect. Dis. 203, 1517–1525 (2011)
Ferreira, C. S. et al. Measles virus infection of alveolar macrophages and dendritic cells precedes spread to lymphatic organs in transgenic mice expressing human signaling lymphocytic activation molecule (SLAM, CD150). J. Virol. 84, 3033–3042 (2010)
Lemon, K. et al. Early target cells of measles virus after aerosol infection of non-human primates. PLoS Pathog. 7, e1001263 (2011)
Leonard, V. H., Hodge, G., Reyes-Del Valle, J., McChesney, M. B. & Cattaneo, R. Measles virus selectively blind to signaling lymphocytic activation molecule (SLAM; CD150) is attenuated and induces strong adaptive immune responses in rhesus monkeys. J. Virol. 84, 3413–3420 (2010)
Tatsuo, H., Ono, N., Tanaka, K. & Yanagi, Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406, 893–897 (2000)
de Swart, R. L. et al. Predominant infection of CD150+ lymphocytes and dendritic cells during measles virus infection of macaques. PLoS Pathog. 3, e178 (2007)
Reymond, N. et al. Nectin4/PRR4, a new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J. Biol. Chem. 276, 43205–43215 (2001)
DeRycke, M. S. et al. Nectin 4 overexpression in ovarian cancer tissues and serum: potential role as a serum biomarker. Am. J. Clin. Pathol. 134, 835–845 (2010)
Fabre-Lafay, S. et al. Nectin-4, a new serological breast cancer marker, is a substrate for tumor necrosis factor-α-converting enzyme (TACE)/ADAM-17. J. Biol. Chem. 280, 19543–19550 (2005)
Takano, A. et al. Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res. 69, 6694–6703 (2009)
Galanis, E. et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 70, 875–882 (2010)
Leonard, V. H. et al. Measles virus blind to its epithelial cell receptor remains virulent in rhesus monkeys but cannot cross the airway epithelium and is not shed. J. Clin. Invest. 118, 2448–2458 (2008)
Wagner, K. W. et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nature Med. 13, 1070–1077 (2007)
Fabre, S. et al. Prominent role of the Ig-like V domain in trans-interactions of nectins. Nectin3 and nectin 4 bind to the predicted C–C'-C′′-D β-strands of the nectin1 V domain. J. Biol. Chem. 277, 27006–27013 (2002)
Brancati, F. et al. Mutations in PVRL4, encoding cell adhesion molecule nectin-4, cause ectodermal dysplasia-syndactyly syndrome. Am. J. Hum. Genet. 87, 265–273 (2010)
Mendelsohn, C. L., Wimmer, E. & Racaniello, V. R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell 56, 855–865 (1989)
Campadelli-Fiume, G., Cocchi, F., Menotti, L. & Lopez, M. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev. Med. Virol. 10, 305–319 (2000)
Noyce, R. S. et al. Tumor cell marker PVRL4 (Nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 7, e1002240 (2011)
Navaratnarajah, C. K. et al. Dynamic interaction of the measles virus hemagglutinin with its receptor signaling lymphocytic activation molecule (SLAM, CD150). J. Biol. Chem. 283, 11763–11771 (2008)
Karp, P. H. et al. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol. Biol. 188, 115–137 (2002)
Tahara, M. et al. Measles virus infects both polarized epithelial and immune cells by using distinctive receptor-binding sites on its hemagglutinin. J. Virol. 82, 4630–4637 (2008)
Welshman, M. D. Measles in the cynomolgus monkey (Macaca fascicularis). Vet. Rec. 124, 184–186 (1989)
Ludlow, M. et al. Wild-type measles virus infection of primary epithelial cells occurs via the basolateral surface without syncytium formation or release of infectious virus. J. Gen. Virol. 91, 971–979 (2010)
Monto, A. S. Interrupting the transmission of respiratory tract infections: theory and practice. Clin. Infect. Dis. 28, 200–204 (1999)
Cattaneo, R., Miest, T., Shashkova, E. V. & Barry, M. A. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nature Rev. Microbiol. 6, 529–540 (2008)
Bergelson, J. M. Intercellular junctional proteins as receptors and barriers to virus infection and spread. Cell Host Microbe 5, 517–521 (2009)
Takeda, M. et al. Recovery of pathogenic measles virus from cloned cDNA. J. Virol. 74, 6643–6647 (2000)
Radecke, F. et al. Rescue of measles viruses from cloned DNA. EMBO J. 14, 5773–5784 (1995)
Reymond, N. et al. DNAM-1 and PVR regulate monocyte migration through endothelial junctions. J. Exp. Med. 199, 1331–1341 (2004)
Acknowledgements
We thank J. Brüning, A. Schnoor Cancio, A. Peterson, I. Meunier and C. Thibault for technical assistance; Y. Yanagi for Vero-hSLAM cells and p(+)MV323-EGFP; T. Stehle for soluble SLAMF1; J. Fournier and G. Kobinger for facilitating the macaque studies; and R. König, D. Schilling-Leiß and T. Miest for discussions. This paper is dedicated to Heinz Schaller by one of his students. This work was supported by grants BMG 2510-FSB-705 to M.D.M.; NIH R01 AI063476 and NIH R01 CA090636 to R.C.; the Roy J. Carver Charitable Trust, Cell Culture Core and Cell Morphology Cores, partially supported by the Center for Gene Therapy for Cystic Fibrosis (NIH P30 DK-54759), and the Cystic Fibrosis Foundation to P.B.M.; and CIHR MOP-66989 and CFI 9488 to V.v.M.; INSERM, Institut Paoli-Calmettes and the Ligue Nationale Contre le Cancer (label 2009–11) to M.L. X.X.W. was supported by a CIHR Master’s Award.
Author information
Authors and Affiliations
Contributions
V.H.J.L. and M.D.M. conceived the project with R.C., who coordinated research. V.H.J.L., S.P., K.M.U. and M.D.M. performed and evaluated screens, and validated nectin-4 as candidate receptor. M.L. contributed purified proteins, antibodies and cell lines, and advised about their use. M.M. and C.K.N. characterized nectin-4 function biochemically and in cells. P.L.S., S.R. and P.B.M. planned and executed experiments with well-differentiated human epithelial sheets. M.F., X.X.W., B.S. and V.v.M. planned and executed monkey infection experiments and their analyses. R.C., M.M. and M.D.M. wrote the paper; V.v.M., K.C., P.B.M. and M.L. edited the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Table 1 and Supplementary Figures 1-8 with legends. (PDF 3948 kb)
Rights and permissions
About this article
Cite this article
Mühlebach, M., Mateo, M., Sinn, P. et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 480, 530–533 (2011). https://doi.org/10.1038/nature10639
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10639
This article is cited by
-
Establishment and characterization of a novel kidney cell line derived from the common bottlenose dolphin
In Vitro Cellular & Developmental Biology - Animal (2023)
-
Metagenomics-enabled reverse-genetics assembly and characterization of myotis bat morbillivirus
Nature Microbiology (2023)
-
Nebulized fusion inhibitory peptide protects cynomolgus macaques from measles virus infection
Nature Communications (2022)
-
Versatility of live-attenuated measles viruses as platform technology for recombinant vaccines
npj Vaccines (2022)
-
Nectin-4: a Novel Therapeutic Target for Skin Cancers
Current Treatment Options in Oncology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.