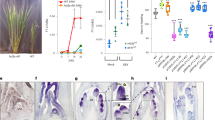
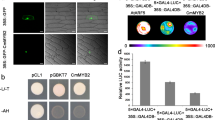
Abstract
Seasonal fluctuations in day length regulate important aspects of plant development such as the flowering transition or, in potato (Solanum tuberosum), the formation of tubers. Day length is sensed by the leaves, which produce a mobile signal transported to the shoot apex or underground stems to induce a flowering transition or, respectively, a tuberization transition. Work in Arabidopsis, tomato and rice (Oryza sativa) identified the mobile FLOWERING LOCUS T (FT) protein as a main component of the long-range ‘florigen’, or flowering hormone, signal1,2,3. Here we show that expression of the Hd3a gene, the FT orthologue in rice, induces strict short-day potato types4 to tuberize in long days. Tuber induction is graft transmissible and the Hd3a–GFP protein is detected in the stolons of grafted plants, transport of the fusion protein thus correlating with tuber formation. We provide evidence showing that the potato floral and tuberization transitions are controlled by two different FT-like paralogues (StSP3D and StSP6A) that respond to independent environmental cues, and show that an autorelay mechanism involving CONSTANS modulates expression of the tuberization-control StSP6A gene.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Turck, F., Fornara, F. & Coupland, G. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu. Rev. Plant Biol. 59, 573–594 (2008)
Lifschitz, E. et al. The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proc. Natl Acad. Sci. USA 103, 6398–6403 (2006)
Zeevaart, J. A. Leaf-produced floral signals. Curr. Opin. Plant Biol. 11, 541–547 (2008)
Jackson, S. D. Multiple signalling pathways control tuber induction in potato. Plant Physiol. 119, 1–8 (1999)
Ewing, E. E. & Struik, P. C. Tuber formation in potato: induction, initiation and growth. Hortic. Rev. (Am. Soc. Hortic. Sci.) 14, 89–197 (1992)
Ishikawa, R. et al. Suppression of the floral activator Hd3a is the principal cause of the night break effect in rice. Plant Cell 17, 3326–3336 (2005)
Lagercrantz, U. At the end of the day: a common molecular mechanism for photoperiod responses in plants? J. Exp. Bot. 60, 2501–2515 (2009)
Rodríguez-Falcón, M., Bou, J. & Prat, S. Seasonal control of tuberization in potato: conserved elements with the flowering response. Annu. Rev. Plant Biol. 57, 151–180 (2006)
Samach, A. et al. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis . Science 288, 1613–1616 (2000)
An, H. et al. CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis . Development 131, 3615–3626 (2004)
Corbesier, L. et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis . Science 316, 1030–1033 (2007)
Jaeger, K. E. & Wigge, P. A. FT protein acts as a long-range signal in Arabidopsis . Curr. Biol. 17, 1050–1054 (2007)
Mathieu, J., Warthmann, N., Kuttner, F. & Schmid, M. Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis . Curr. Biol. 17, 1055–1060 (2007)
Yano, M. et al. Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12, 2473–2484 (2000)
Kojima, S. et al. Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol. 43, 1096–1105 (2002)
Hayama, R., Yokoi, S., Tamaki, S., Yano, M. & Shimamoto, K. Adaptation of photoperiodic control pathways produces short-day flowering in rice. Nature 422, 719–722 (2003)
Martinez-Garcia, J. F., Virgos-Soler, A. & Prat, S. Control of photoperiod-regulated tuberization in potato by the Arabidopsis flowering-time gene CONSTANS. Proc. Natl Acad. Sci. USA 99, 15211–15216 (2002)
Tamaki, S., Matsuo, S., Wong, H. L., Yokoi, S. & Shimamoto, K. Hd3a protein is a mobile flowering signal in rice. Science 316, 1033–1036 (2007)
Carmel-Goren, L., Liu, Y. S., Lifschitz, E. & Zamir, D. The SELF-PRUNING gene family in tomato. Plant Mol. Biol. 52, 1215–1222 (2003)
The Potato Genome Sequencing Consortium . Genome sequence and analysis of the tuber crop potato. Nature 475, 189–195 (2011)
Jackson, S. D., Heyer, A., Dietze, J. & Prat, S. Phytochrome B mediates the photoperiodic control of tuber formation in potato. Plant J. 9, 159–166 (1996)
Ben-Naim, O. et al. The CCAAT binding factor can mediate interactions between CONSTANS-like proteins and DNA. Plant J. 46, 462–476 (2006)
Lifschitz, E. & Eshed, Y. Universal florigenic signals triggered by FT homologues regulate growth and flowering cycles in perennial day-neutral tomato. J. Exp. Bot. 57, 3405–3414 (2006)
Pin, P. A. et al. An antagonistic pair of FT homologs mediates the control of flowering time in sugar beet. Science 330, 1397–1400 (2010)
Shalit, A. et al. The flowering hormone florigen functions as a general systemic regulator of growth and termination. Proc. Natl Acad. Sci. USA 106, 8392–8397 (2009)
Chailakhyan, M. K., Yanina, L. I., Davedzhiyan, A. G. & Lotova, G. N. Photoperiodism and tuber formation in grafting of tobacco onto potato. Dokl. Akad. Nauk SSSR 257, 1276–1280 (1981)
Abe, M. et al. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309, 1052–1056 (2005)
Wigge, P. A. et al. Integration of spatial and temporal information during floral induction in Arabidopsis . Science 309, 1056–1059 (2005)
Kloosterman, B. et al. StGA2ox1 is induced prior to stolon swelling and controls GA levels during potato tuber development. Plant J. 52, 362–373 (2007)
Bohlenius, H. et al. CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312, 1040–1043 (2006)
Nakagawa, T. et al. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 104, 34–41 (2007)
Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 (2001)
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative C T method. Nature Protocols 3, 1101–1108 (2008)
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 - ΔΔ C T method. Methods 25, 402–408 (2001)
Kloosterman, B. et al. Genes driving potato tuber initiation and growth: identification based on transcriptional changes using the POCI array. Funct. Integr. Genomics 8, 329–340 (2008)
Smyth, G. K. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3, 3 (2004)
Smyth, G. K. & Speed, T. Normalization of cDNA microarray data. Methods 31, 265–273 (2003)
Tamura, K., Dudley, J., Nei, M. & Kumar, S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24, 1596–1599 (2007)
Saitou, N. & Nei, M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425 (1987)
Acknowledgements
We thank J. Paz-Ares, G. Bryan and C. Bachem for their comments on the manuscript. We also thank S. Yokoi for his help in this work. This research was supported by grants from the Spanish MCyT and the EU-SOL European Union Integrated Project. Support by the JSPS and CSIC under the Japan–Spain research cooperative programme and Grants-in-Aid for Scientific Research on Priority Areas of the MEXT of Japan are also acknowledged. C.N. was recipient of an I3P postdoctoral contract from the CSIC.
Author information
Authors and Affiliations
Contributions
C.N., J.A.A., E.C.-O., C.A.C., J.S. and S.P. performed experiments and analysed the results. S.T. and K.S. provided the rice Hd3a construct and performed the Hd3a protein mobility studies. S.P. designed the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Text 1-6, additional references, Supplementary Figures 1-13 with legends and Supplementary Tables 1-3. (PDF 531 kb)
Rights and permissions
About this article
Cite this article
Navarro, C., Abelenda, J., Cruz-Oró, E. et al. Control of flowering and storage organ formation in potato by FLOWERING LOCUS T. Nature 478, 119–122 (2011). https://doi.org/10.1038/nature10431
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10431
This article is cited by
-
Rose FT homologous gene overexpression affects flowering and vegetative development behavior in two different rose genotypes
Plant Cell, Tissue and Organ Culture (PCTOC) (2024)
-
BEL transcription factors in prominent Solanaceae crops: the missing pieces of the jigsaw in plant development
Planta (2024)
-
Biological Activity of Anti-bolting Compound, α-(7Z,10Z,13Z)-hexadeca-7,10,13-trienoic Acid Monoglyceride to Reduce the Endogenous Amount of Gibberellins
Journal of Plant Growth Regulation (2023)
-
Temperature Effects on Tuber Production and Carbohydrates Partitioning in Different Cultivars during Consecutive Stages of Potato (Solanum tuberosum L.) Growth
Potato Research (2023)
-
Analysis of PEBP Genes in Saffron Identifies a Flowering Locus T Homologue Involved in Flowering Regulation
Journal of Plant Growth Regulation (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.