Abstract
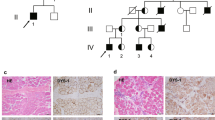
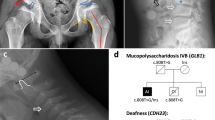
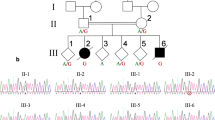
Fukuyama muscular dystrophy (FCMD; MIM253800), one of the most common autosomal recessive disorders in Japan, was the first human disease found to result from ancestral insertion of a SINE-VNTR-Alu (SVA) retrotransposon into a causative gene1,2,3. In FCMD, the SVA insertion occurs in the 3′ untranslated region (UTR) of the fukutin gene. The pathogenic mechanism for FCMD is unknown, and no effective clinical treatments exist. Here we show that aberrant messenger RNA (mRNA) splicing, induced by SVA exon-trapping, underlies the molecular pathogenesis of FCMD. Quantitative mRNA analysis pinpointed a region that was missing from transcripts in patients with FCMD. This region spans part of the 3′ end of the fukutin coding region, a proximal part of the 3′ UTR and the SVA insertion. Correspondingly, fukutin mRNA transcripts in patients with FCMD and SVA knock-in model mice were shorter than the expected length. Sequence analysis revealed an abnormal splicing event, provoked by a strong acceptor site in SVA and a rare alternative donor site in fukutin exon 10. The resulting product truncates the fukutin carboxy (C) terminus and adds 129 amino acids encoded by the SVA. Introduction of antisense oligonucleotides (AONs) targeting the splice acceptor, the predicted exonic splicing enhancer and the intronic splicing enhancer prevented pathogenic exon-trapping by SVA in cells of patients with FCMD and model mice, rescuing normal fukutin mRNA expression and protein production. AON treatment also restored fukutin functions, including O-glycosylation of α-dystroglycan (α-DG) and laminin binding by α-DG. Moreover, we observe exon-trapping in other SVA insertions associated with disease (hypercholesterolemia4, neutral lipid storage disease5) and human-specific SVA insertion in a novel gene. Thus, although splicing into SVA is known6,7,8, we have discovered in human disease a role for SVA-mediated exon-trapping and demonstrated the promise of splicing modulation therapy as the first radical clinical treatment for FCMD and other SVA-mediated diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Data deposits
The patient fukutin and a chimpanzee mRNA sequences are deposited in GenBank/European Molecular Biology Laboratory/DNA Data Bank of Japan under accession numbers AB609007 and AB627340, respectively.
References
Toda, T. et al. Localization of a gene for Fukuyama type congenital muscular dystrophy to chromosome 9q31–33. Nature Genet. 5, 283–286 (1993)
Kobayashi, K. et al. An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature 394, 388–392 (1998)
Watanabe, M. et al. Founder SVA retrotransposal insertion in Fukuyama-type congenital muscular dystrophy and its origin in Japanese and Northeast Asian populations. Am. J. Med. Genet. A. 138, 344–348 (2005)
Wilund, K. R. et al. Molecular mechanisms of autosomal recessive hypercholesterolemia. Hum. Mol. Genet. 11, 3019–3030 (2002)
Akman, H. O. et al. Neutral lipid storage disease with subclinical myopathy due to a retrotransposal insertion in the PNPLA2 gene. Neuromuscul. Disord. 20, 397–402 (2010)
Hancks, D. C. et al. Exon-trapping mediated by the human retrotransposon SVA. Genome Res. 19, 1983–1991 (2009)
Damert, A. et al. 5′-Transducing SVA retrotransposon groups spread efficiently throughout the human genome. Genome Res. 19, 1992–2008 (2009)
Bantysh, O. B. & Buzdin, A. A. Novel family of human transposable elements formed due to fusion of the first exon of gene MAST2 with retrotransposon SVA. Biochemistry (Mosc.) 74, 1393–1399 (2009)
Michele, D. E. et al. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature 418, 417–422 (2002)
Barresi, R. & Campbell, K. P. Dystroglycan: from biosynthesis to pathogenesis of human disease. J. Cell Sci. 119, 199–207 (2006)
Strichman-Almashanu, L. Z. et al. Retroposed copies of the HMG genes: a window to genome dynamics. Genome Res. 13, 800–812 (2003)
Ostertag, E. M. et al. SVA elements are nonautonomous retrotransposons that cause disease in humans. Am. J. Hum. Genet. 73, 1444–1451 (2003)
Bennett, E. A. et al. Natural genetic variation caused by transposable elements in humans. Genetics 168, 933–951 (2004)
Wang, H. et al. SVA elements: a hominid-specific retroposon family. J. Mol. Biol. 354, 994–1007 (2005)
Hancks, D. C. et al. Retrotransposition of marked SVA elements by human L1s in cultured cells. Hum. Mol. Genet. 20, 3386–3400 (2011)
Kanagawa, M. et al. Residual laminin-binding activity and enhanced dystroglycan glycosylation by LARGE in novel model mice to dystroglycanopathy. Hum. Mol. Genet. 18, 621–631 (2009)
Hancks, D. C. & Kazazian, H. H., Jr SVA retrotransposons: evolution and genetic instability. Semin. Cancer Biol. 20, 234–245 (2010)
Wu, B. et al. Octa-guanidine morpholino restores dystrophin expression in cardiac and skeletal muscles and ameliorates pathology in dystrophic mdx mice. Mol. Ther. 17, 864–871 (2009)
Barresi, R. et al. LARGE can functionally bypass α-dystroglycan glycosylation defects in distinct congenital muscular dystrophies. Nature Med. 10, 696–703 (2004)
Lander, E. S. et al. Initial sequencing and analysis of the human genome. Nature 409, 860–921 (2001)
Kazazian, H. H., Jr Mobile elements: drivers of genome evolution. Science 303, 1626–1632 (2004)
Cordaux, R. & Batzer, M. A. The impact of retrotransposons on human genome evolution. Nature Rev. Genet. 10, 691–703 (2009)
Hassoun, H. et al. A novel mobile element inserted in the α spectrin gene: spectrin dayton. A truncated α spectrin associated with hereditary elliptocytosis. J. Clin. Invest. 94, 643–648 (1994)
Rohrer, J. et al. Unusual mutations in Btk: an insertion, a duplication, an inversion, and four large deletions. Clin. Immunol. 90, 28–37 (1999)
Legoix, P. et al. Molecular characterization of germline NF2 gene rearrangements. Genomics 65, 62–66 (2000)
Makino, S. et al. Reduced neuron-specific expression of the TAF1 gene is associated with X-linked dystonia-parkinsonism. Am. J. Hum. Genet. 80, 393–406 (2007)
O’Brien, S. et al. Randomized phase III trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J. Clin. Oncol. 25, 1114–1120 (2007)
Crooke, S. T. et al. Vitravene—another piece in the mosaic. Antisense Nucleic Acid Drug Dev. 8, vii–viii. (1998)
Lu, Q. L. et al. Functional amounts of dystrophin produced by skipping the mutated exon in the mdx dystrophic mouse. Nature Med. 9, 1009–1014 (2003)
Alter, J. et al. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nature Med. 12, 175–177 (2006)
Yokota, T. et al. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann. Neurol. 65, 667–676 (2009)
Takeda, S. et al. Fukutin is required for maintenance of muscle integrity, cortical histiogenesis and normal eye development. Hum. Mol. Genet. 12, 1449–1459 (2003)
Jurka, J. Repbase Update: a database and an electronic journal of repetitive elements. Trends Genet. 9, 418–420 (2000)
Acknowledgements
We thank S. Nakagawa, K. Ohno, S. Tsujino, N. Taniguchi, and I. Nonaka for comments; M. Okabe and A. Kawai for generating the ES cell line from knock-in model mice; Y. Motoyoshi and J. C. Cohen for providing patients’ samples; W. Sako and Y. Izumi for sending patients’ samples; I. Mizuta, T. Mure, M. Furukawa, K. Kaneshiro, Y. Dainin and all laboratory members for technical support; and J. Logan for editing the manuscript. We thank the GAIN for providing chimpanzee brain samples. This work was supported by an Intramural Research Grant (20B-13) for Neurological and Psychiatric Disorders from the National Center of Neurology and Psychiatry (to T.T.), the Global COE Program (Frontier Biomedical Science Underlying Organelle Network Biology) (to T.T., M.T.-I. and M.K.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Grants-in-Aid for Scientific Research (A) (23249049 to T.T.), and Young Scientists (A) (21689030 to K.K.) and (B) (20790980 to M.T.-I.) from the Japan Society for the Promotion of Science, and the Takeda Science Foundation (to K.K.).
Author information
Authors and Affiliations
Contributions
M.T.-I., K.K., M.K. and T.T. designed the study. M.T.-I. performed most of the experiments. K.K. developed a system to detect endogenous fukutin protein. M.K. performed biochemical analysis of VMO-injected mice. C.Y. produced the fukutin cDNA constructs for transfection experiments. K.M., T.O., and A.K. performed analyses of AON treatment in mice and various cell types. H.K., T.Y. and S.T. provided intellectual input. H.O.A., S.D. and R.K., provided patients’ samples. M.T.-I., K.K. and T.T. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains supplementary Figures 1-13 with legends and Supplementary Table 1. (PDF 3522 kb)
Rights and permissions
About this article
Cite this article
Taniguchi-Ikeda, M., Kobayashi, K., Kanagawa, M. et al. Pathogenic exon-trapping by SVA retrotransposon and rescue in Fukuyama muscular dystrophy. Nature 478, 127–131 (2011). https://doi.org/10.1038/nature10456
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10456
This article is cited by
-
A SINE-VNTR-Alu at the LRIG2 locus is associated with proximal and distal gene expression in CRISPR and population models
Scientific Reports (2024)
-
Retrotransposon insertion as a novel mutational cause of spinal muscular atrophy
Human Genetics (2023)
-
The landscape of aging
Science China Life Sciences (2022)
-
Efficacy of steroid therapy for Fukuyama congenital muscular dystrophy
Scientific Reports (2021)
-
SVA retrotransposon insertion in exon of MMR genes results in aberrant RNA splicing and causes Lynch syndrome
European Journal of Human Genetics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.