Abstract
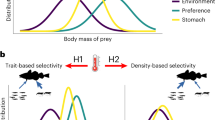
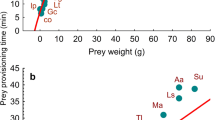
A central challenge for predators is achieving positive energy balance when prey are spatially and temporally heterogeneous. Ecological heterogeneity produces evolutionary trade-offs in the physiological design of predators; this is because the ability to capitalize on pulses of food abundance requires high capacity for food-processing, yet maintaining such capacity imposes energetic costs that are taxing during periods of food scarcity1,2. Recent advances in physiology show that when variation in foraging opportunities is predictable, animals may adjust energetic trade-offs by rapidly modulating their digestive system to track variation in foraging opportunities1. However, it is increasingly recognized that foraging opportunities for animals are unpredictable3, which should favour animals that maintain a capacity for food-processing that exceeds average levels of consumption (loads)2,4. Despite this basic principle of quantitative evolutionary design, estimates of digestive load:capacity ratios in wild animals are virtually non-existent1. Here we provide an extensive assessment of load:capacity ratios for the digestive systems of predators in the wild, compiling 639 estimates across 38 species of fish. We found that piscine predators typically maintain the physiological capacity to feed at daily rates 2–3 times higher than what they experience on average. A numerical simulation of the trade-off between food-processing capacity and metabolic cost suggests that the observed level of physiological opportunism is profitable only if predator–prey encounters, and thus predator energy budgets, are far more variable in nature than currently assumed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Piersma, T. & van Gils, J. A. The Flexible Phenotype (Oxford Univ. Press, 2011)
Diamond, J. Quantitative evolutionary design. J. Physiol. (Lond.) 542, 337–345 (2002)
Humphries, N. E. et al. Environmental context explains Levy and Brownian movement patterns of marine predators. Nature 465, 1066–1069 (2010)
Gans, C. Momentarily excessive construction as the basis for protoadaptation. Evolution 33, 227–233 (1979)
Ritchie, E. G. & Johnson, C. N. Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 12, 982–998 (2009)
Walters, C. J. M. & Martell, S. J. D. Fisheries Ecology and Management (Princeton Univ. Press, 2004)
Kauffman, M. J. et al. Landscape heterogeneity shapes predation in a newly restored predator-prey system. Ecol. Lett. 10, 690–700 (2007)
Armstrong, J. B. et al. Thermal heterogeneity mediates the effects of pulsed subsidies across a landscape. Ecology 91, 1445–1454 (2010)
Klaassen, M., Lindstrom, A. & Zijlstra, R. Composition of fuel stores and digestive limitations to fuel deposition rate in the long-distance migratory thrush nightingale, Luscinia luscinia . Physiol. Zool. 70, 125–133 (1997)
Dupont-Prinet, A. et al. Physiological mechanisms underlying a trade-off between growth rate and tolerance of feed deprivation in the European sea bass (Dicentrarchus labrax). J. Exp. Biol. 213, 1143–1152 (2010)
Weiner, J. Physiological limits to sustainable energy budgets in birds and mammals: ecological implications. Trends Ecol. Evol. 7, 384–388 (1992)
Weibel, E. R., Taylor, C. R. & Hoppeler, H. The concept of symmorphosis: a testable hypothesis of structure-function relationship. Proc. Natl Acad. Sci. USA 88, 10357–10361 (1991)
Kersten, M. & Visser, W. The rate of food processing in the oystercatcher: food intake and energy expenditure constrained by a digestive bottleneck. Funct. Ecol. 10, 440–448 (1996)
Essington, T. E., Hodgson, J. R. & Kitchell, J. F. Role of satiation in the functional response of a piscivore, largemouth bass (Micropterus salmoides). Can. J. Fish. Aquat. Sci. 57, 548–556 (2000)
Holling, C. S. The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can. Entomol. 91, 293–320 (1959)
Kitchell, J. F., Stewart, D. J. & Weininger, D. Applications of a bioenergetics model to yellow perch (Perca-flavescens) and walleye (Stizostedion-vitreum-vitreum). J. Fish. Res. Board Can. 34, 1922–1935 (1977)
Fish. Bioenergetics 3.0 (University of Wisconsin-Madison Centre for Limnology/Wisconsin Sea Grant Institute, 1997)
Elliott, J. M. & Persson, L. Estimation of daily rates of food consumption for fish. J. Anim. Ecol. 47, 977–991 (1978)
Kitchell, J. F. et al. Predator-prey dynamics in an ecosystem context. J. Fish Biol. 45, 209–226 (1994)
Secor, S. M., Stein, E. D. & Diamond, J. Rapid up-regulation of snake intestine in response to feeding: a new model of intestinal adaptation. Am. J. Physiol. 266, G695–G705 (1994)
Breck, J. E. Foraging theory and piscivorous fish: are forage fish just big zooplankton? Trans. Am. Fish. Soc. 122, 902–911 (1993)
DeAngelis, D. L. & Gross, L. J. Individual-based Models and Approaches in Ecology. Populations, Communities, and Ecosystems (Chapman and Hall, 1992)
Huey, R. B., Pianka, E. R. & Vitt, L. J. How often do lizards “run on empty”? Ecology 82, 1–7 (2001)
Arrington, D. A., Winemiller, K. O., Loftus, W. F. & Akin, S. How often do fishes “run on empty”? Ecology 83, 2145–2151 (2002)
Jeschke, J. M. When carnivores are “full and lazy”. Oecologia 152, 357–364 (2007)
Brodin, A. & Clark, C. in Foraging (eds Stephen, D. S., Brown, J. S. & Ydenberg, R. C. ) 221–269 (Univ. Chicago Press, 2007)
Hofmann, R. R. Evolutionary steps of ecophysiological adaptation and diversification of ruminants: a comparative view of their digestive system. Oecologia 78, 443–457 (1989)
Schindler, D. E. & Eby, L. A. Stoichiometry of fishes and their prey: implications for nutrient recycling. Ecology 78, 1816–1831 (1997)
Yang, L. H., Bastow, J. L., Spence, K. O. & Wright, A. N. What can we learn from resource pulses? Ecology 89, 621–634 (2008)
McWilliams, S. R. & Karasov, W. H. Phenotypic flexibility in digestive system structure and function in migratory birds and its ecological significance. Comp. Biochem. Physiol. A 128, 577–593 (2001)
Rice, J. A. & Cochran, P. A. Independent evaluation of a bioenergetics model for largemouth bass. Ecology 65, 732–739 (1984)
Beauchamp, D. A., Stewart, D. J. & Thomas, G. L. corroboration of a bioenergetics model for sockeye salmon. Trans. Am. Fish. Soc. 118, 597–607 (1989)
R Development Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna); available at 〈http://www.R-project.org〉 (2010)
Scott, D. W . Multivariate Density Estimation: Theory, Practice, and Visualization (Wiley, 1992)
Stevens, E. D. & Devlin, R. H. Intestinal morphology in growth hormone transgenic coho salmon. J. Fish Biol. 56, 191–195 (2000)
Farrell, A. P. et al. Gut blood flow in fish during exercise and severe hypercapnia. Comp. Biochem. Physiol. A 128, 549–561 (2001)
Acknowledgements
We thank T. Essington, R. Huey, T. Reed, A. Walters, V. Sturtevant and A. Armstrong for comments on this manuscript. We also thank the following people for contributing to this project: J. Kitchell, O. Jensen, E. Ward, D. Beauchamp, B. Chasco, A. Farrell, P. Bisson and J. Kershner. This work was supported by the Gordon and Betty Moore Foundation, the US National Science Foundation and the University of Washington School of Aquatic and Fishery Sciences.
Author information
Authors and Affiliations
Contributions
J.B.A. and D.E.S. contributed to each stage of the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Figure 1 with legend, Supplementary Table 1 and additional references. (PDF 201 kb)
PowerPoint slides
Rights and permissions
About this article
Cite this article
Armstrong, J., Schindler, D. Excess digestive capacity in predators reflects a life of feast and famine. Nature 476, 84–87 (2011). https://doi.org/10.1038/nature10240
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10240
This article is cited by
-
Seasonal changes in invertebrate drift: effects of declining summer flows on prey abundance for drift-feeding fishes
Hydrobiologia (2022)
-
The importance of warm habitat to the growth regime of cold-water fishes
Nature Climate Change (2021)
-
A first look at the metabolic rate of Greenland sharks (Somniosus microcephalus) in the Canadian Arctic
Scientific Reports (2020)
-
Ecological Consequences of Animal Migration: Prey Partial Migration Affects Predator Ecology and Prey Communities
Ecosystems (2020)
-
Energy depletion of embryos and yolk-sac feeding larvae of the liparid snailfish Careproctus pallidus (Vaillant 1888)
Polar Biology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.