Abstract
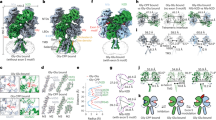
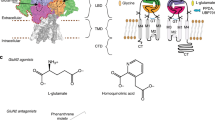
Since it was discovered that the anti-hypertensive agent ifenprodil has neuroprotective activity through its effects on NMDA (N-methyl-D-aspartate) receptors1, a determined effort has been made to understand the mechanism of action and to develop improved therapeutic compounds on the basis of this knowledge2,3,4. Neurotransmission mediated by NMDA receptors is essential for basic brain development and function5. These receptors form heteromeric ion channels and become activated after concurrent binding of glycine and glutamate to the GluN1 and GluN2 subunits, respectively. A functional hallmark of NMDA receptors is that their ion-channel activity is allosterically regulated by binding of small compounds to the amino-terminal domain (ATD) in a subtype-specific manner. Ifenprodil and related phenylethanolamine compounds, which specifically inhibit GluN1 and GluN2B NMDA receptors6,7, have been intensely studied for their potential use in the treatment of various neurological disorders and diseases, including depression, Alzheimer’s disease and Parkinson’s disease2,4. Despite considerable enthusiasm, mechanisms underlying the recognition of phenylethanolamines and ATD-mediated allosteric inhibition remain limited owing to a lack of structural information. Here we report that the GluN1 and GluN2B ATDs form a heterodimer and that phenylethanolamine binds at the interface between GluN1 and GluN2B, rather than within the GluN2B cleft. The crystal structure of the heterodimer formed between the GluN1b ATD from Xenopus laevis and the GluN2B ATD from Rattus norvegicus shows a highly distinct pattern of subunit arrangement that is different from the arrangements observed in homodimeric non-NMDA receptors and reveals the molecular determinants for phenylethanolamine binding. Restriction of domain movement in the bi-lobed structure of the GluN2B ATD, by engineering of an inter-subunit disulphide bond, markedly decreases sensitivity to ifenprodil, indicating that conformational freedom in the GluN2B ATD is essential for ifenprodil-mediated allosteric inhibition of NMDA receptors. These findings pave the way for improving the design of subtype-specific compounds with therapeutic value for neurological disorders and diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gotti, B. et al. Ifenprodil and SL 82.0715 as cerebral anti-ischemic agents. I. Evidence for efficacy in models of focal cerebral ischemia. J. Pharmacol. Exp. Ther. 247, 1211–1221 (1988)
Koller, M. & Urwyler, S. Novel N-methyl-D-aspartate receptor antagonists: a review of compounds patented since 2006. Expert Opin. Ther. Pat. 20, 1683–1702 (2010)
Hansen, K. B., Furukawa, H. & Traynelis, S. F. Control of assembly and function of glutamate receptors by the amino-terminal domain. Mol. Pharmacol. 78, 535–549 (2010)
Mony, L., Kew, J. N., Gunthorpe, M. J. & Paoletti, P. Allosteric modulators of NR2B-containing NMDA receptors: molecular mechanisms and therapeutic potential. Br. J. Pharmacol. 157, 1301–1317 (2009)
Traynelis, S. F. et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 62, 405–496 (2010)
Gallagher, M. J., Huang, H., Pritchett, D. B. & Lynch, D. R. Interactions between ifenprodil and the NR2B subunit of the N-methyl-D-aspartate receptor. J. Biol. Chem. 271, 9603–9611 (1996)
Williams, K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol. Pharmacol. 44, 851–859 (1993)
Ewald, R. C. & Cline, H. T. Cloning and phylogenetic analysis of NMDA receptor subunits NR1, NR2A and NR2B in Xenopus laevis tadpoles. Front. Mol. Neurosci. 2, 4 (2009)
Schmidt, C. & Hollmann, M. Molecular and functional characterization of Xenopus laevis N-methyl-D-aspartate receptors. Mol. Cell. Neurosci. 42, 116–127 (2009)
Karakas, E., Simorowski, N. & Furukawa, H. Structure of the zinc-bound amino-terminal domain of the NMDA receptor NR2B subunit. EMBO J. 28, 3910–3920 (2009)
Kumar, J., Schuck, P., Jin, R. & Mayer, M. L. The N-terminal domain of GluR6-subtype glutamate receptor ion channels. Nature Struct. Mol. Biol. 16, 631–638 (2009)
Jin, R. et al. Crystal structure and association behaviour of the GluR2 amino-terminal domain. EMBO J. 28, 1812–1823 (2009)
Clayton, A. et al. Crystal structure of the GluR2 amino-terminal domain provides insights into the architecture and assembly of ionotropic glutamate receptors. J. Mol. Biol. 392, 1125–1132 (2009)
Sobolevsky, A. I., Rosconi, M. P. & Gouaux, E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature 462, 745–756 (2009)
Gielen, M., Siegler Retchless, B., Mony, L., Johnson, J. W. & Paoletti, P. Mechanism of differential control of NMDA receptor activity by NR2 subunits. Nature 459, 703–707 (2009)
Rachline, J., Perin-Dureau, F., Le Goff, A., Neyton, J. & Paoletti, P. The micromolar zinc-binding domain on the NMDA receptor subunit NR2B. J. Neurosci. 25, 308–317 (2005)
Malherbe, P. et al. Identification of critical residues in the amino terminal domain of the human NR2B subunit involved in the RO 25-6981 binding pocket. J. Pharmacol. Exp. Ther. 307, 897–905 (2003)
Masuko, T. et al. A regulatory domain (R1–R2) in the amino terminus of the N-methyl-D-aspartate receptor: effects of spermine, protons, and ifenprodil, and structural similarity to bacterial leucine/isoleucine/valine binding protein. Mol. Pharmacol. 55, 957–969 (1999)
Perin-Dureau, F., Rachline, J., Neyton, J. & Paoletti, P. Mapping the binding site of the neuroprotectant ifenprodil on NMDA receptors. J. Neurosci. 22, 5955–5965 (2002)
Lee, C. H. & Gouaux, E. Amino terminal domains of the NMDA receptor are organized as local heterodimers. PLoS ONE 6, e19181 (2011)
Adams, P. D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D 58, 1948–1954 (2002)
Fitzgerald, D. J. et al. Protein complex expression by using multigene baculoviral vectors. Nature Methods 3, 1021–1032 (2006)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
de La Fortelle, E. & Bricogne, G. Maximum-likelihood heavy-atom parameter refinement for multiple isomorphous replacement and multiwavelength anomalous diffraction methods. Methods Enzymol. 276, 472–494 (1997)
Cohen, S. X. et al. Towards complete validated models in the next generation of ARP/wARP. Acta Crystallogr. D 60, 2222–2229 (2004)
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Cryst. 40, 658–674 (2007)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Schuck, P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and Lamm equation modeling. Biophys. J. 78, 1606–1619 (2000)
Furukawa, H., Singh, S. K., Mancusso, R. & Gouaux, E. Subunit arrangement and function in NMDA receptors. Nature 438, 185–192 (2005)
Acknowledgements
We thank the staff at X25 and X29 at the National Synchrotron Light Source for beamline support. M. Mayer is thanked for comments on this work. We also thank D. Raleigh for the use of analytical ultracentrifugation. GluN1 clones from Xenopus laevis were gifts from M. Hollmann and H. Cline. This work was supported by NIH MH085926, the Alzheimer’s Association and a donation from the Fox family (to H.F.). H.F. was also funded by a scientist development grant from the American Heart Association. E.K. is supported by a NARSAD Lieber Young Investigator Award.
Author information
Authors and Affiliations
Contributions
The project was initiated by E.K. and H.F. All of the experiments were designed by E.K. and H.F. Crystallographic studies, isothermal calorimetry and analytical ultracentrifugation were carried out by E.K. Electrophysiology and crosslinking experiments were conducted by H.F. Technical support was given by N.S. The manuscript was written by H.F. and E.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-12 with legends, Supplementary Tables 1-2 and additional references. (PDF 1376 kb)
Rights and permissions
About this article
Cite this article
Karakas, E., Simorowski, N. & Furukawa, H. Subunit arrangement and phenylethanolamine binding in GluN1/GluN2B NMDA receptors. Nature 475, 249–253 (2011). https://doi.org/10.1038/nature10180
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10180
This article is cited by
-
Synthesis of polysubstituted azepanes by dearomative ring expansion of nitroarenes
Nature Chemistry (2024)
-
Unveiling the molecular basis of lobeline's allosteric regulation of NMDAR: insights from molecular modeling
Scientific Reports (2023)
-
Isomerization of bioactive acylhydrazones triggered by light or thiols
Nature Chemistry (2023)
-
Progresses in GluN2A-containing NMDA Receptors and their Selective Regulators
Cellular and Molecular Neurobiology (2023)
-
Structural insights into binding of therapeutic channel blockers in NMDA receptors
Nature Structural & Molecular Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.