Abstract
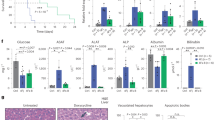
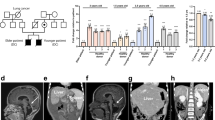
The differentiation of patient-derived induced pluripotent stem cells (iPSCs) to committed fates such as neurons, muscle and liver is a powerful approach for understanding key parameters of human development and disease1,2,3,4,5,6. Whether undifferentiated iPSCs themselves can be used to probe disease mechanisms is uncertain. Dyskeratosis congenita is characterized by defective maintenance of blood, pulmonary tissue and epidermal tissues and is caused by mutations in genes controlling telomere homeostasis7,8. Short telomeres, a hallmark of dyskeratosis congenita, impair tissue stem cell function in mouse models, indicating that a tissue stem cell defect may underlie the pathophysiology of dyskeratosis congenita9,10. Here we show that even in the undifferentiated state, iPSCs from dyskeratosis congenita patients harbour the precise biochemical defects characteristic of each form of the disease and that the magnitude of the telomere maintenance defect in iPSCs correlates with clinical severity. In iPSCs from patients with heterozygous mutations in TERT, the telomerase reverse transcriptase, a 50% reduction in telomerase levels blunts the natural telomere elongation that accompanies reprogramming. In contrast, mutation of dyskerin (DKC1) in X-linked dyskeratosis congenita severely impairs telomerase activity by blocking telomerase assembly and disrupts telomere elongation during reprogramming. In iPSCs from a form of dyskeratosis congenita caused by mutations in TCAB1 (also known as WRAP53), telomerase catalytic activity is unperturbed, yet the ability of telomerase to lengthen telomeres is abrogated, because telomerase mislocalizes from Cajal bodies to nucleoli within the iPSCs. Extended culture of DKC1-mutant iPSCs leads to progressive telomere shortening and eventual loss of self-renewal, indicating that a similar process occurs in tissue stem cells in dyskeratosis congenita patients. These findings in iPSCs from dyskeratosis congenita patients reveal that undifferentiated iPSCs accurately recapitulate features of a human stem cell disease and may serve as a cell-culture-based system for the development of targeted therapeutics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007)
Rashid, S. T. et al. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J. Clin. Invest. 120, 3127–3136 (2010)
Liu, G. H. et al. Recapitulation of premature ageing with iPSCs from Hutchinson–Gilford progeria syndrome. Nature advance online publication. 10.1038/nature09879 (23 February 2011)
Soldner, F. et al. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell 136, 964–977 (2009)
Dimos, J. T. et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 321, 1218–1221 (2008)
Maehr, R. et al. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc. Natl Acad. Sci. USA 106, 15768–15773 (2009)
Walne, A. J. & Dokal, I. Advances in the understanding of dyskeratosis congenita. Br. J. Haematol. 145, 164–172 (2009)
Alter, B. P. et al. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br. J. Haematol. 150, 179–188 (2010)
Lee, H. W. et al. Essential role of mouse telomerase in highly proliferative organs. Nature 392, 569–574 (1998)
Allsopp, R. C., Morin, G. B., DePinho, R., Harley, C. B. & Weissman, I. L. Telomerase is required to slow telomere shortening and extend replicative lifespan of HSCs during serial transplantation. Blood 102, 517–520 (2003)
Bessler, M., Wilson, D. B. & Mason, P. J. Dyskeratosis congenita. FEBS Lett. 584, 3831–3838 (2010)
Mitchell, J. R., Wood, E. & Collins, K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature 402, 551–555 (1999)
Zhong, F. et al. Disruption of telomerase trafficking by TCAB1 mutation causes dyskeratosis congenita. Genes Dev. 25, 11–16 (2011)
Alter, B. P. et al. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood 110, 1439–1447 (2007)
Agarwal, S. et al. Telomere elongation in induced pluripotent stem cells from dyskeratosis congenita patients. Nature 464, 292–296 (2010)
Marion, R. M. et al. Telomeres acquire embryonic stem cell characteristics in induced pluripotent stem cells. Cell Stem Cell 4, 141–154 (2009)
Stadtfeld, M., Maherali, N., Breault, D. T. & Hochedlinger, K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell 2, 230–240 (2008)
Yoshida, Y., Takahashi, K., Okita, K., Ichisaka, T. & Yamanaka, S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell 5, 237–241 (2009)
Wong, J. M. & Collins, K. Telomerase RNA level limits telomere maintenance in X-linked dyskeratosis congenita. Genes Dev. 20, 2848–2858 (2006)
Zaug, A. J., Podell, E. R., Nandakumar, J. & Cech, T. R. Functional interaction between telomere protein TPP1 and telomerase. Genes Dev. 24, 613–622 (2010)
Vulliamy, T. et al. Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nature Genet. 36, 447–449 (2004)
Armanios, M. et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc. Natl Acad. Sci. USA 102, 15960–15964 (2005)
Venteicher, A. S. et al. A human telomerase holoenzyme protein required for Cajal body localization and telomere synthesis. Science 323, 644–648 (2009)
Tycowski, K. T., Shu, M. D., Kukoyi, A. & Steitz, J. A. A conserved WD40 protein binds the Cajal body localization signal of scaRNP particles. Mol. Cell 34, 47–57 (2009)
Lundblad, V. & Szostak, J. W. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell 57, 633–643 (1989)
Matera, A. G., Terns, R. M. & Terns, M. P. Non-coding RNAs: lessons from the small nuclear and small nucleolar RNAs. Nature Rev. Mol. Cell Biol. 8, 209–220 (2007)
Byrne, J. A., Nguyen, H. N. & Reijo Pera, R. A. Enhanced generation of induced pluripotent stem cells from a subpopulation of human fibroblasts. PLoS ONE 4, e7118 (2009)
Sommer, C. A. et al. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells 27, 543–549 (2009)
Tomlinson, R. L. et al. Telomerase reverse transcriptase is required for the localization of telomerase RNA to Cajal bodies and telomeres in human cancer cells. Mol. Biol. Cell 19, 3793–3800 (2008)
Middleman, E. J., Choi, J., Venteicher, A. S., Cheung, P. & Artandi, S. E. Regulation of cellular immortalization and steady-state levels of the telomerase reverse transcriptase through its carboxy-terminal domain. Mol. Cell. Biol. 26, 2146–2159 (2006)
Cristofari, G. & Lingner, J. Telomere length homeostasis requires that telomerase levels are limiting. EMBO J. 25, 565–574 (2006)
Lei, M., Zaug, A. J., Podell, E. R. & Cech, T. R. Switching human telomerase on and off with hPOT1 protein in vitro. J. Biol. Chem. 280, 20449–20456 (2005)
Wang, F. et al. The POT1–TPP1 telomere complex is a telomerase processivity factor. Nature 445, 506–510 (2007)
Venteicher, A. S., Meng, Z., Mason, P. J., Veenstra, T. D. & Artandi, S. E. Identification of ATPases pontin and reptin as telomerase components essential for holoenzyme assembly. Cell 132, 945–957 (2008)
Mochizuki, Y., He, J., Kulkarni, S., Bessler, M. & Mason, P. J. Mouse dyskerin mutations affect accumulation of telomerase RNA and small nucleolar RNA, telomerase activity, and ribosomal RNA processing. Proc. Natl Acad. Sci. USA 101, 10756–10761 (2004)
Seabright, M. Rapid banding technique for human chromosomes. Lancet 2, 971–972 (1971)
Deb-Rinker, P., Ly, D., Jezierski, A., Sikorska, M. & Walker, P. R. Sequential DNA methylation of the Nanog and Oct-4 upstream regions in human NT2 cells during neuronal differentiation. J. Biol. Chem. 280, 6257–6260 (2005)
Acknowledgements
L.F.Z.B. is the recipient of a Pew Fellowship. M.F.P. and K.T.X. are the recipients of NSF Graduate Research Fellowships. F.L.Z. was supported by A*STAR, Singapore. We thank the patients for their valuable contributions and L. Leathwood for study support. This work was supported, in part, by the intramural research program of the Division of Cancer Epidemiology and Genetics, NCI, NIH; by a CIRM Shared Research Laboratory grant to R.R.P.; and by grants from the NCI, NIA, NHLBI and CIRM to S.E.A.
Author information
Authors and Affiliations
Contributions
L.F.Z.B., M.F.P., F.L.Z and S.E.A. designed the experiments and analysed the data; L.F.Z.B., M.F.P., F.L.Z., H.N.N., K.T.X., A.J.Z., S.M.C., J.C., V.S. and A.C. performed the experiments; N.G., M.W., B.P.A., T.R.C., S.A.S. and R.A.R.P. analysed the data; N.G., B.P.A and S.A.S. collected patients’ material; L.F.Z.B. and S.E.A. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-15 with legends, Supplementary Tables 1-2 and additional references. (PDF 1585 kb)
Rights and permissions
About this article
Cite this article
Batista, L., Pech, M., Zhong, F. et al. Telomere shortening and loss of self-renewal in dyskeratosis congenita induced pluripotent stem cells. Nature 474, 399–402 (2011). https://doi.org/10.1038/nature10084
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10084
This article is cited by
-
New insights into the epitranscriptomic control of pluripotent stem cell fate
Experimental & Molecular Medicine (2022)
-
“Cutting the Mustard” with Induced Pluripotent Stem Cells: An Overview and Applications in Healthcare Paradigm
Stem Cell Reviews and Reports (2022)
-
Biallelic mutations in WRAP53 result in dysfunctional telomeres, Cajal bodies and DNA repair, thereby causing Hoyeraal–Hreidarsson syndrome
Cell Death & Disease (2020)
-
Regulation of human telomerase in homeostasis and disease
Nature Reviews Molecular Cell Biology (2020)
-
Three-dimensional modeling of human neurodegeneration: brain organoids coming of age
Molecular Psychiatry (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.