Abstract
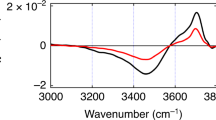
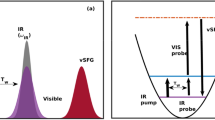
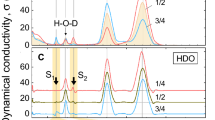
The air–water interface is perhaps the most common liquid interface. It covers more than 70 per cent of the Earth’s surface and strongly affects atmospheric, aerosol and environmental chemistry. The air–water interface has also attracted much interest as a model system that allows rigorous tests of theory, with one fundamental question being just how thin it is. Theoretical studies have suggested a surprisingly short ‘healing length’ of about 3 ångströms (1 Å = 0.1 nm), with the bulk-phase properties of water recovered within the top few monolayers1,2,3. However, direct experimental evidence has been elusive owing to the difficulty of depth-profiling the liquid surface on the ångström scale. Most physical, chemical and biological properties of water, such as viscosity, solvation, wetting and the hydrophobic effect, are determined by its hydrogen-bond network. This can be probed by observing the lineshape of the OH-stretch mode, the frequency shift of which is related to the hydrogen-bond strength4,5,6. Here we report a combined experimental and theoretical study of the air–water interface using surface-selective heterodyne-detected vibrational sum frequency spectroscopy to focus on the ‘free OD’ transition found only in the topmost water layer. By using deuterated water and isotopic dilution to reveal the vibrational coupling mechanism, we find that the free OD stretch is affected only by intramolecular coupling to the stretching of the other OD group on the same molecule. The other OD stretch frequency indicates the strength of one of the first hydrogen bonds encountered at the surface; this is the donor hydrogen bond of the water molecule straddling the interface, which we find to be only slightly weaker than bulk-phase water hydrogen bonds. We infer from this observation a remarkably fast onset of bulk-phase behaviour on crossing from the air into the water phase.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Townsend, R. M., Gryko, J. & Rice, S. A. Structure of the liquid vapor interface of water. J. Chem. Phys. 82, 4391–4392 (1985)
Morita, A. & Hynes, J. T. A theoretical analysis of the sum frequency generation spectrum of the water surface. Chem. Phys. 258, 371–390 (2000)
Taylor, R. S., Dang, L. X. & Garrett, B. C. Molecular dynamics simulations of the liquid/vapor interface of SPC/E water. J. Phys. Chem. 100, 11720–11725 (1996)
Rey, R., Moller, K. B. & Hynes, J. T. Hydrogen bond dynamics in water and ultrafast infrared spectroscopy. J. Phys. Chem. A 106, 11993–11996 (2002)
Lawrence, C. P. & Skinner, J. L. Ultrafast infrared spectroscopy probes hydrogen-bonding dynamics in liquid water. Chem. Phys. Lett. 369, 472–477 (2003)
Fecko, C. J., Eaves, J. D., Loparo, J. J., Tokmakoff, A. & Geissler, P. L. Ultrafast hydrogen-bond dynamics in the infrared spectroscopy of water. Science 301, 1698–1702 (2003)
Richmond, G. L. Molecular bonding and interactions at aqueous surfaces as probed by vibrational sum frequency spectroscopy. Chem. Rev. 102, 2693–2724 (2002)
Ostroverkhov, V., Waychunas, G. A. & Shen, Y. R. New information on water interfacial structure revealed by phase-sensitive surface spectroscopy. Phys. Rev. Lett. 94, 046102 (2005)
Sovago, M., Campen, R. K., Bakker, H. J. & Bonn, M. Hydrogen bonding strength of interfacial water determined with surface sum-frequency generation. Chem. Phys. Lett. 470, 7–12 (2009)
Stiopkin, I. V., Jayathilake, H. D., Bordenyuk, A. N. & Benderskii, A. V. Heterodyne-detected vibrational sum frequency generation spectroscopy. J. Am. Chem. Soc. 130, 2271–2275 (2008)
Gan, W., Wu, D., Zhang, Z., Feng, R. R. & Wang, H. F. Polarization and experimental configuration analyses of sum frequency generation vibrational spectra, structure, and orientational motion of the air/water interface. J. Chem. Phys. 124, 114705 (2006)
Nihonyanagi, S., Yamaguchi, S. & Tahara, T. Direct evidence for orientational flip-flop of water molecules at charged interfaces: a heterodyne-detected vibrational sum frequency generation study. J. Chem. Phys. 130, 204704 (2009)
Auer, B. M. & Skinner, J. L. Vibrational sum-frequency spectroscopy of the liquid/vapor interface for dilute HOD in D2O. J. Chem. Phys. 129, 214705 (2008)
Corcelli, S. A., Lawrence, C. P. & Skinner, J. L. Combined electronic structure/molecular dynamics approach for ultrafast infrared spectroscopy of dilute HOD in liquid H2O and D2O. J. Chem. Phys. 120, 8107–8117 (2004)
Nagata, Y. & Mukamel, S. Vibrational sum-frequency generation spectroscopy at the water/lipid interface: molecular dynamics simulation study. J. Am. Chem. Soc. 132, 6434–6442 (2010)
Ishiyama, T. & Morita, A. Vibrational spectroscopic response of intermolecular orientational correlation at the water surface. J. Phys. Chem. C 113, 16299–16302 (2009)
Du, Q., Superfine, R., Freysz, E. & Shen, Y. R. Vibrational spectroscopy of water at the vapor water interface. Phys. Rev. Lett. 70, 2313–2316 (1993)
Du, Q., Freysz, E. & Shen, Y. R. Surface vibrational spectroscopic studies of hydrogen-bonding and hydrophobicity. Science 264, 826–828 (1994)
Skinner, J. L., Auer, B. M. & Lin, Y. S. Vibrational line shapes, spectral diffusion, and hydrogen bonding in liquid water. Adv. Chem. Phys. 142, 59–103 (2009)
Woutersen, S. & Bakker, H. J. Resonant intermolecular transfer of vibrational energy in liquid water. Nature 402, 507–509 (1999)
Raymond, E. A., Tarbuck, T. L., Brown, M. G. & Richmond, G. L. Hydrogen-bonding interactions at the vapor/water interface investigated by vibrational sum-frequency spectroscopy of HOD/H2O/D2O mixtures and molecular dynamics simulations. J. Phys. Chem. B 107, 546–556 (2003)
Tian, C. S. & Shen, Y. R. Isotopic dilution study of the water/vapor interface by phase-sensitive sum-frequency vibrational spectroscopy. J. Am. Chem. Soc. 131, 2790–2791 (2009)
Lepetit, L., Cheriaux, G. & Joffre, M. Linear techniques of phase measurement by femtosecond spectral interferometry for applications in spectroscopy. J. Opt. Soc. Am. B 12, 2467–2474 (1995)
Stiopkin, I. V., Jayathilake, H. D., Weeraman, C. & Benderskii, A. V. Temporal effects on spectroscopic line shapes, resolution, and sensitivity of the broad-band sum frequency generation. J. Chem. Phys. 132, 234503 (2010)
Shimanouchi, T. Tables of Molecular Vibrational Frequencies Consolidated Vol. I (National Bureau of Standards, 1972)
Auer, B. M. & Skinner, J. L. Vibrational sum-frequency spectroscopy of the water liquid/vapor interface. J. Phys. Chem. B 113, 4125–4130 (2009)
Corcelli, S. A. & Skinner, J. L. Infrared and Raman line shapes of dilute HOD in liquid H2O and D2O from 10 to 90 degrees C. J. Phys. Chem. A 109, 6154–6165 (2005)
Loparo, J. J., Roberts, S. T., Nicodemus, R. A. & Tokmakoff, A. Variation of the transition dipole moment across the OH stretching band of water. Chem. Phys. 341, 218–229 (2007)
Barker, E. F. & Sleator, W. W. The infrared spectrum of heavy water. J. Chem. Phys. 3, 660–663 (1935)
Max, J. J. & Chapados, C. Isotope effects in liquid water by infrared spectroscopy. J. Chem. Phys. 116, 4626–4642 (2002)
Acknowledgements
The experiments presented here were supported by the NSF CAREER grant number CHE-0449720 (I.V.S., C.W., F.Y.S. and A.V.B.). J.L.S. thanks the NSF and the DOE for support from grants CHE-0750307 and DE-FG02-09ER16110, respectively.
Author information
Authors and Affiliations
Contributions
I.V.S., C.W., F.Y.S. and A.V.B. constructed the spectroscopic set-up, executed the experiments, and performed the analysis of the spectroscopic data. P.A.P. and J.L.S. carried out molecular dynamics simulations and calculation of the spectra. All authors discussed the results and contributed to the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Text, Supplementary Figures 1-5 with legends and additional references. (PDF 851 kb)
Rights and permissions
About this article
Cite this article
Stiopkin, I., Weeraman, C., Pieniazek, P. et al. Hydrogen bonding at the water surface revealed by isotopic dilution spectroscopy. Nature 474, 192–195 (2011). https://doi.org/10.1038/nature10173
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10173
This article is cited by
-
Unified picture of vibrational relaxation of OH stretch at the air/water interface
Nature Communications (2024)
-
Surface stratification determines the interfacial water structure of simple electrolyte solutions
Nature Chemistry (2024)
-
Faster chemistry at surfaces
Nature Chemistry (2021)
-
Asymmetric response of interfacial water to applied electric fields
Nature (2021)
-
Observation of an exotic state of water in the hydrophilic nanospace of porous coordination polymers
Communications Chemistry (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.