Abstract
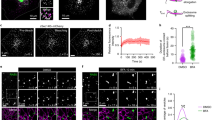
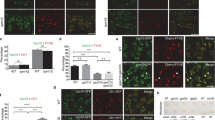
How the directionality of vesicle traffic is achieved remains an important unanswered question in cell biology. The Sec23p/Sec24p coat complex sorts the fusion machinery (SNAREs) into vesicles as they bud from the endoplasmic reticulum (ER). Vesicle tethering to the Golgi begins when the tethering factor TRAPPI binds to Sec23p. Where the coat is released and how this event relates to membrane fusion is unknown. Here we use a yeast transport assay to demonstrate that an ER-derived vesicle retains its coat until it reaches the Golgi. A Golgi-associated kinase, Hrr25p (CK1δ orthologue), then phosphorylates the Sec23p/Sec24p complex. Coat phosphorylation and dephosphorylation are needed for vesicle fusion and budding, respectively. Additionally, we show that Sec23p interacts in a sequential manner with different binding partners, including TRAPPI and Hrr25p, to ensure the directionality of ER–Golgi traffic and prevent the back-fusion of a COPII vesicle with the ER. These events are conserved in mammalian cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Jahn, R. & Scheller, R. H. SNAREs—engines for membrane fusion. Nature Rev. Mol. Cell Biol. 7, 631–643 (2006)
Lewis, M. J., Rayner, J. C. & Pelham, H. R. A novel SNARE complex implicated in vesicle fusion with the endoplasmic reticulum. EMBO J. 16, 3017–3024 (1997)
Mossessova, E., Bickford, L. C. & Goldberg, J. SNARE selectivity of the COPII coat. Cell 114, 483–495 (2003)
Lee, M. C. S., Miller, E. A., Goldberg, J., Orci, L. & Schekman, R. Bi-directional protein transport between the ER and Golgi. Annu. Rev. Cell Dev. Biol. 20, 87–123 (2004)
Whyte, J. R. C. & Munro, S. Vesicle tethering complexes in membrane traffic. J. Cell Sci. 115, 2627–2637 (2002)
Cai, H. et al. TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature 445, 941–944 (2007)
Barlowe, C. et al. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77, 895–907 (1994)
Flanagan, J. J. & Barlowe, C. Cysteine-disulfide cross-linking to monitor SNARE complex assembly during endoplasmic reticulum-Golgi transport. J. Biol. Chem. 281, 2281–2288 (2006)
Dudognon, P., Maeder-Garavaglia, C., Carpentier, J. & Paccaud, J. Regulation of a COPII component by cytosolic O-glycosylation during mitosis. FEBS Lett. 561, 44–50 (2004)
Milne, D. M., Looby, P. & Meek, D. W. Catalytic activity of protein kinase CK1δ (casein kinase 1δ) is essential for its normal subcellular localization. Exp. Cell Res. 263, 43–54 (2001)
Yu, S. & Roth, M. G. Casein kinase I regulates membrane binding by ARF GAP1. Mol. Biol. Cell 13, 2559–2570 (2002)
Murakami, A., Kimura, K. & Nakano, A. The inactive form of a yeast casein kinase I suppresses the secretory defect of the sec12 mutant. J. Biol. Chem. 274, 3804–3810 (1999)
Lusk, C. P. et al. Nup53p is a target of two mitotic kinases, Cdk1p and Hrr25p. Traffic 8, 647–660 (2007)
Kafadar, K. A., Zhu, H., Snyder, M. & Cyert, M. S. Negative regulation of calcineurin signaling by Hrr25p, a yeast homolog of casein kinase I. Genes Dev. 17, 2698–2708 (2003)
Bi, X., Corpina, R. A. & Goldberg, J. Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature 419, 271–277 (2002)
Mashhoon, N. et al. Crystal structure of a conformation-selective casein kinase-1 inhibitor. J. Biol. Chem. 275, 20052–20060 (2000)
Xu, D. & Hay, J. C. Reconstitution of COPII vesicle fusion to generate a pre-Golgi intermediate compartment. J. Cell Biol. 167, 997–1003 (2004)
Schweizer, A. et al. Identification of an intermediate compartment involved in protein transport from endoplasmic reticulum to Golgi apparatus. Eur. J. Cell Biol. 53, 185–196 (1990)
Bielli, A. et al. Regulation of Sar1 NH2 terminus by GTP binding and hydrolysis promotes membrane deformation to control COPII vesicle fission. J. Cell Biol. 171, 919–924 (2005)
Lee, M. C. S. et al. Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell 122, 605–617 (2005)
Chin, H. F. et al. Kinetic analysis of the guanine nucleotide exchange activity of TRAPP, a multimeric Ypt1p exchange factor. J. Mol. Biol. 389, 275–288 (2009)
Salama, N. R., Chuang, J. S. & Schekman, R. W. Sec31 encodes an essential component of the COPII coat required for transport vesicle budding from the endoplasmic reticulum. Mol. Biol. Cell 8, 205–217 (1997)
Guttman, M. et al. Interactions of the NPXY microdomains of the low density lipoprotein receptor-related protein 1. Proteomics 9, 5016–5028 (2009)
Gimeno, R. E., Espenshade, P. & Kaiser, C. A. COPII coat subunit interactions: Sec24p and Sec23p bind to adjacent regions of Sec16p. Mol. Biol. Cell 7, 1815–1823 (1996)
Fromme, J. C. et al. The genetic basis of a craniofacial disease provides insight into COPII coat assembly. Dev. Cell 13, 623–634 (2007)
Diao, A., Frost, L., Morohashi, Y. & Lowe, M. Coordination of golgin tethering and SNARE assembly: GM130 binds syntaxin 5 in a p115-regulated manner. J. Biol. Chem. 283, 6957–6967 (2008)
Saraste, J. & Svensson, K. Distribution of the intermediate elements operating in ER to Golgi transport. J. Cell Sci. 100, 415–430 (1991)
Hay, J. C. et al. Localization, dynamics, and protein interactions reveal distinct roles for ER and Golgi SNAREs. J. Cell Biol. 141, 1489–1502 (1998)
Bentley, M. et al. Vesicular calcium regulates coat retention, fusogenicity, and size of pre-Golgi intermediates. Mol. Biol. Cell 21, 1033–1046 (2010)
Acknowledgements
We thank R. Schekman, M. Cyert, L. Miller, B. Glick, M. Lowe and E. Mizuno-Yamasaki for strains, constructs, purified protein and antibody; K. Reinisch for discussions; S. Chen, H. Cai and M. Garcia-Marcos for advice; W. Zhou and A. Lougheed for technical assistance. This work was supported by the Howard Hughes Medical Institute. Salary support for S.F.-N., D.B. and S.M. was provided by the Howard Hughes Medical Institute, P.G. was funded by the Burroughs Wellcome Fund and M.G. by the SRP Super Fund Research Program. Work at the University of Montana was supported by National Institutes of Health grant GM-059378 (to J.C.H.) and the National Institutes of Health COBRE Center grant RR-015583.
Author information
Authors and Affiliations
Contributions
C.L., D.B., S.M., D.N. and J.H. performed experiments and analysed data. M.G. did the mass analysis and P.G. did the computational modelling and analysed data. S.F.-N. directed the project, analysed data and wrote the paper. C.L., D.B., J.H. and P.G. co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-5 with legends and an additional reference. (PDF 596 kb)
Rights and permissions
About this article
Cite this article
Lord, C., Bhandari, D., Menon, S. et al. Sequential interactions with Sec23 control the direction of vesicle traffic. Nature 473, 181–186 (2011). https://doi.org/10.1038/nature09969
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09969
This article is cited by
-
Genipin prevents alpha-synuclein aggregation and toxicity by affecting endocytosis, metabolism and lipid storage
Nature Communications (2023)
-
Changes in hepatic triglyceride content with the activation of ER stress and increased FGF21 secretion during pregnancy
Nutrition & Metabolism (2021)
-
It is all about the process(ing): P-body granules and the regulation of signal transduction
Current Genetics (2020)
-
Consequences of mutations in the genes of the ER export machinery COPII in vertebrates
Cell Stress and Chaperones (2020)
-
COPII-dependent ER export in animal cells: adaptation and control for diverse cargo
Histochemistry and Cell Biology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.