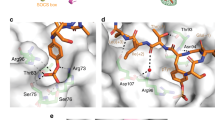
Abstract
Inhibitor of κB (IκB) kinase (IKK) phosphorylates IκB proteins, leading to their degradation and the liberation of nuclear factor κB for gene transcription. Here we report the crystal structure of IKKβ in complex with an inhibitor, at a resolution of 3.6 Å. The structure reveals a trimodular architecture comprising the kinase domain, a ubiquitin-like domain (ULD) and an elongated, α-helical scaffold/dimerization domain (SDD). Unexpectedly, the predicted leucine zipper and helix–loop–helix motifs do not form these structures but are part of the SDD. The ULD and SDD mediate a critical interaction with IκBα that restricts substrate specificity, and the ULD is also required for catalytic activity. The SDD mediates IKKβ dimerization, but dimerization per se is not important for maintaining IKKβ activity and instead is required for IKKβ activation. Other IKK family members, IKKα, TBK1 and IKK-i, may have a similar trimodular architecture and function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hayden, M. S. & Ghosh, S. Shared principles in NF-κB signaling. Cell 132, 344–362 (2008)
Vallabhapurapu, S. & Karin, M. Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 27, 693–733 (2009)
Scheidereit, C. IκB kinase complexes: gateways to NF-κB activation and transcription. Oncogene 25, 6685–6705 (2006)
Karin, M. Nuclear factor-κB in cancer development and progression. Nature 441, 431–436 (2006)
Chen, Z. J., Parent, L. & Maniatis, T. Site-specific phosphorylation of IκBα by a novel ubiquitination-dependent protein kinase activity. Cell 84, 853–862 (1996)
DiDonato, J. A. et al. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature 388, 548–554 (1997)
Mercurio, F. et al. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science 278, 860–866 (1997)
Woronicz, J. D. et al. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science 278, 866–870 (1997)
Yamaoka, S. et al. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell 93, 1231–1240 (1998)
Zandi, E. et al. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell 91, 243–252 (1997)
Rothwarf, D. M., Zandi, E., Natoli, G. & Karin, M. IKK-γ is an essential regulatory subunit of the IκB kinase complex. Nature 395, 297–300 (1998)
Hacker, H. & Karin, M. Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006, re13 (2006)
Zandi, E., Chen, Y. & Karin, M. Direct phosphorylation of IκB by IKKα and IKKβ: discrimination between free and NF-κB-bound substrate. Science 281, 1360–1363 (1998)
Sato, S. et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nature Immunol. 6, 1087–1095 (2005)
Liu, H. H., Xie, M., Schneider, M. D. & Chen, Z. J. Essential role of TAK1 in thymocyte development and activation. Proc. Natl Acad. Sci. USA 103, 11677–11682 (2006)
Tang, E. D. et al. Roles for homotypic interactions and transautophosphorylation in IκB kinase (IKKβ) activation. J. Biol. Chem. 278, 38566–38570 (2003); erratum. 278, 49661 (2003)
Knighton, D. R. et al. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science 253, 407–414 (1991)
Dikic, I., Wakatsuki, S. & Walters, K. J. Ubiquitin-binding domains — from structures to functions. Nature Rev. Mol. Cell Biol. 10, 659–671 (2009)
Zheng, J. et al. 2.2 Å refined crystal structure of the catalytic subunit of cAMP-dependent protein kinase complexed with MnATP and a peptide inhibitor. Acta Crystallogr. D 49, 362–365 (1993)
Bossemeyer, D. et al. Phosphotransferase and substrate binding mechanism of the cAMP-dependent protein kinase catalytic subunit from porcine heart as deduced from the 2.0 Å structure of the complex with Mn2+ adenylyl imidodiphosphate and inhibitor peptide PKI(5–24). EMBO J. 12, 849–859 (1993)
Xu, R. M., Carmel, G., Kuret, J. & Cheng, X. Structural basis for selectivity of the isoquinoline sulfonamide family of protein kinase inhibitors. Proc. Natl Acad. Sci. USA 93, 6308–6313 (1996)
Sicheri, F., Moarefi, I. & Kuriyan, J. Crystal structure of the Src family tyrosine kinase Hck. Nature 385, 602–609 (1997)
Noble, M. E., Endicott, J. A. & Johnson, L. N. Protein kinase inhibitors: insights into drug design from structure. Science 303, 1800–1805 (2004)
Nolen, B., Taylor, S. & Ghosh, G. Regulation of protein kinases; controlling activity through activation segment conformation. Mol. Cell 15, 661–675 (2004)
Jeffrey, P. D. et al. Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature 376, 313–320 (1995)
May, M. J. et al. A novel ubiquitin-like domain in IκB kinase β is required for functional activity of the kinase. J. Biol. Chem. 279, 45528–45539 (2004)
Goldsmith, E. J. et al. Substrate and docking interactions in serine/threonine protein kinases. Chem. Rev. 107, 5065–5081 (2007)
Brown, K. et al. Structural basis for the interaction of TAK1 kinase with its activating protein TAB1. J. Mol. Biol. 354, 1013–1020 (2005)
Ikeda, F. et al. Involvement of the ubiquitin-like domain of TBK1/IKK-i kinases in regulation of IFN-inducible genes. EMBO J. 26, 3451–3462 (2007)
Kato, T., Jr, Delhase, M., Hoffmann, A. & Karin, M. CK2 is a C-terminal IκB kinase responsible for NF-κB activation during the UV response. Mol. Cell 12, 829–839 (2003)
Barroga, C. F., Stevenson, J. K., Schwarz, E. M. & Verma, I. M. Constitutive phosphorylation of I kappa B alpha by casein kinase II. Proc. Natl Acad. Sci. USA 92, 7637–7641 (1995)
Shaul, J. D., Farina, A. & Huxford, T. The human IKKβ subunit kinase domain displays CK2-like phosphorylation specificity. Biochem. Biophys. Res. Commun. 374, 592–597 (2008)
Lo, Y. C. et al. Structural basis for recognition of diubiquitins by NEMO. Mol. Cell 33, 602–615 (2009)
Rahighi, S. et al. Specific recognition of linear ubiquitin chains by NEMO is important for NF-κB activation. Cell 136, 1098–1109 (2009)
Rushe, M. et al. Structure of a NEMO/IKK-associating domain reveals architecture of the interaction site. Structure 16, 798–808 (2008)
Bagnéris, C. et al. Crystal structure of a vFlip-IKKγ complex: insights into viral activation of the IKK signalosome. Mol. Cell 30, 620–631 (2008)
Cordier, F. et al. Solution structure of NEMO zinc finger and impact of an anhidrotic ectodermal dysplasia with immunodeficiency-related point mutation. J. Mol. Biol. 377, 1419–1432 (2008)
Remenyi, A., Good, M. C. & Lim, W. A. Docking interactions in protein kinase and phosphatase networks. Curr. Opin. Struct. Biol. 16, 676–685 (2006)
Kallunki, T., Deng, T., Hibi, M. & Karin, M. c-Jun can recruit JNK to phosphorylate dimerization partners via specific docking interactions. Cell 87, 929–939 (1996)
Wu, G. et al. Structure of a β-TrCP1-Skp1-β-catenin complex: destruction motif binding and lysine specificity of the SCF(β-TrCP1) ubiquitin ligase. Mol. Cell 11, 1445–1456 (2003)
Ikeda, S. et al. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3β and beta-catenin and promotes GSK-3β-dependent phosphorylation of β-catenin. EMBO J. 17, 1371–1384 (1998)
Hart, M. J. et al. Downregulation of β-catenin by human Axin and its association with the APC tumor suppressor, β-catenin and GSK3β. Curr. Biol. 8, 573–581 (1998)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
Schneider, T. R. & Sheldrick, G. M. Substructure solution with SHELXD. Acta Crystallogr. D 58, 1772–1779 (2002)
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Bricogne, G. et al. Generation, representation and flow of phase information in structure determination: recent developments in and around SHARP 2.0. Acta Crystallogr. D 59, 2023–2030 (2003)
Jones, T. A., Zou, J.-Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for building models in electron density maps and the location of errors in those models. Acta Crystallogr. A 47, 110–119 (1991)
Winn, M. D., Murshudov, G. N. & Papiz, M. Z. Macromolecular TLS refinement in REFMAC at moderate resolutions. Methods Enzymol. 374, 300–321 (2003)
Holm, L. & Sander, C. Dali: a network tool for protein structure comparison. Trends Biochem. Sci. 20, 478–480 (1995)
DeLano, W. L. PyMOL Molecular Viewer 〈http://www.pymol.org〉 (2002)
Acknowledgements
We thank K. Rajashankar and N. Sukumar for data collection at the NE-CAT of APS, B. Schwer for help with the kinase assay, P. Gaillard for help with the chemistry and G. Ahlsen, L. Shapiro and B. Honig for the ultracentrifugation experiments. This work was supported by the National Institutes of Health (H.W. and M.K.), the American Heart Association (G.X. and Y.-C.L.) and the Cancer Research Institute (Y.-C.L.). M.K. is an American Cancer Society Research Professor.
Author information
Authors and Affiliations
Contributions
G.X. cloned, expressed, purified, crystallized and determined the crystal structure of xIKKβ and performed experiments to determine Km. Y.-C.L. cloned, expressed, purified and crystallized hIKKβ and performed pull-down experiments and kinase assays using phospho-IκBα antibody. Q.L. expressed the hIKKβ mutants in insect cells. G.N. and X.W. performed transfection, immunoprecipitation and kinase assays and M.K. supervised these experiments. H.W. supervised the project. G.X and H.W. made the figures and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Tables 1-3 and Supplementary Figures 1-10 with legends. (PDF 13697 kb)
Rights and permissions
About this article
Cite this article
Xu, G., Lo, YC., Li, Q. et al. Crystal structure of inhibitor of κB kinase β. Nature 472, 325–330 (2011). https://doi.org/10.1038/nature09853
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09853
This article is cited by
-
Virtual structure-based docking and molecular dynamics of FDA-approved drugs for the identification of potential IKKB inhibitors possessing dopaminergic activity in Alzheimer’s disease
Chemical Papers (2023)
-
A central role of IKK2 and TPL2 in JNK activation and viral B-cell transformation
Nature Communications (2020)
-
ACT001 modulates the NF-κB/MnSOD/ROS axis by targeting IKKβ to inhibit glioblastoma cell growth
Journal of Molecular Medicine (2020)
-
HUWE1 E3 ligase promotes PINK1/PARKIN-independent mitophagy by regulating AMBRA1 activation via IKKα
Nature Communications (2018)
-
Repurposing Thioridazine (TDZ) as an anti-inflammatory agent
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.