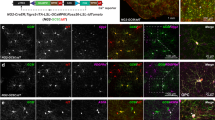
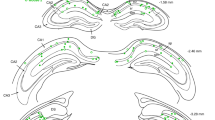
Abstract
Electrical activity has been shown to regulate development in a variety of species and in various structures1, including the retina2,3,4, spinal cord5,6 and cortex5. Within the mammalian cortex specifically, the development of dendrites and commissural axons in pyramidal cells is activity-dependent7,8. However, little is known about the developmental role of activity in the other major cortical population of neurons, the GABA-producing interneurons. These neurons are morphologically and functionally heterogeneous and efforts over the past decade have focused on determining the mechanisms that contribute to this diversity9,10,11. It was recently discovered that 30% of all cortical interneurons arise from a relatively novel source within the ventral telencephalon, the caudal ganglionic eminence (CGE)11,12. Owing to their late birth date, these interneurons populate the cortex only after the majority of other interneurons and pyramidal cells are already in place and have started to functionally integrate. Here we demonstrate in mice that for CGE-derived reelin (Re)-positive and calretinin (Cr)-positive (but not vasoactive intestinal peptide (VIP)-positive) interneurons12,13, activity is essential before postnatal day 3 for correct migration, and that after postnatal day 3, glutamate-mediated activity controls the development of their axons and dendrites. Furthermore, we show that the engulfment and cell motility 1 gene (Elmo1)14, a target of the transcription factor distal-less homeobox 1 (Dlx1)15, is selectively expressed in Re+ and Cr+ interneurons and is both necessary and sufficient for activity-dependent interneuron migration. Our findings reveal a selective requirement for activity in shaping the cortical integration of specific neuronal subtypes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
21 April 2011
Reference 9 was substituted.
References
Blankenship, A. G. & Feller, M. B. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nature Rev. Neurosci. 11, 18–29 (2010)
Wong, R. O., Chernjavsky, A., Smith, S. J. & Shatz, C. J. Early functional neural networks in the developing retina. Nature 374, 716–718 (1995)
Penn, A. A., Riquelme, P. A., Feller, M. B. & Shatz, C. J. Competition in retinogeniculate patterning driven by spontaneous activity. Science 279, 2108–2112 (1998)
Huberman, A. D. et al. Architecture and activity-mediated refinement of axonal projections from a mosaic of genetically identified retinal ganglion cells. Neuron 59, 425–438 (2008)
Spitzer, N. C. Electrical activity in early neuronal development. Nature 444, 707–712 (2006)
Root, C. M., Velazquez-Ulloa, N. A., Monsalve, G. C., Minakova, E. & Spitzer, N. C. Embryonically expressed GABA and glutamate drive electrical activity regulating neurotransmitter specification. J. Neurosci. 28, 4777–4784 (2008)
Cancedda, L., Fiumelli, H., Chen, K. & Poo, M. M. Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo . J. Neurosci. 27, 5224–5235 (2007)
Wang, C. L. et al. Activity-dependent development of callosal projections in the somatosensory cortex. J. Neurosci. 27, 11334–11342 (2007)
Bortone, D. & Polleux, F. KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron 62, 53–71 (2009)
Ascoli, G. A. et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nature Rev. Neurosci. 9, 557–568 (2008)
Batista-Brito, R. & Fishell, G. The developmental integration of cortical interneurons into a functional network. Curr. Top. Dev. Biol. 87, 81–118 (2009)
Miyoshi, G. et al. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J. Neurosci. 30, 1582–1594 (2010)
Karube, F., Kubota, Y. & Kawaguchi, Y. Axon branching and synaptic bouton phenotypes in GABAergic nonpyramidal cell subtypes. J. Neurosci. 24, 2853–2865 (2004)
Gumienny, T. L. et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell 107, 27–41 (2001)
Cobos, I., Borello, U. & Rubenstein, J. L. Dlx transcription factors promote migration through repression of axon and dendrite growth. Neuron 54, 873–888 (2007)
Allene, C. & Cossart, R. Early NMDA receptor-driven waves of activity in the developing neocortex: physiological or pathological network oscillations? J. Physiol. (Lond.) 588, 83–91 (2010)
Garaschuk, O., Linn, J., Eilers, J. & Konnerth, A. Large-scale oscillatory calcium waves in the immature cortex. Nature Neurosci. 3, 452–459 (2000)
Dupont, E., Hanganu, I. L., Kilb, W., Hirsch, S. & Luhmann, H. J. Rapid developmental switch in the mechanisms driving early cortical columnar networks. Nature 439, 79–83 (2006)
Stenman, J., Toresson, H. & Campbell, K. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J. Neurosci. 23, 167–174 (2003)
Yu, C. R. et al. Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron 42, 553–566 (2004)
Yang, J. W., Hanganu-Opatz, I. L., Sun, J. J. & Luhmann, H. J. Three patterns of oscillatory activity differentially synchronize developing neocortical networks in vivo . J. Neurosci. 29, 9011–9025 (2009)
Khazipov, R. & Luhmann, H. J. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 29, 414–418 (2006)
McCabe, A. K., Chisholm, S. L., Picken-Bahrey, H. L. & Moody, W. J. The self-regulating nature of spontaneous synchronized activity in developing mouse cortical neurones. J. Physiol. (Lond.) 577, 155–167 (2006)
Manent, J. B., Jorquera, I., Ben-Ari, Y., Aniksztejn, L. & Represa, A. Glutamate acting on AMPA but not NMDA receptors modulates the migration of hippocampal interneurons. J. Neurosci. 26, 5901–5909 (2006)
Stone, T. W. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol. Rev. 45, 309–379 (1993)
Anderson, S. A., Eisenstat, D. D., Shi, L. & Rubenstein, J. L. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science 278, 474–476 (1997)
Wonders, C. P. & Anderson, S. A. The origin and specification of cortical interneurons. Nature Rev. Neurosci. 7, 687–696 (2006)
Cobos, I. et al. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nature Neurosci. 8, 1059–1068 (2005)
Ravichandran, K. S. & Lorenz, U. Engulfment of apoptotic cells: signals for a good meal. Nature Rev. Immunol. 7, 964–974 (2007)
Park, D. et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450, 430–434 (2007)
Saito, T. In vivo electroporation in the embryonic mouse central nervous system. Nature Protocols 1, 1552–1558 (2006)
Butt, S. J. et al. The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron 59, 722–732 (2008)
Tamamaki, N. et al. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J. Comp. Neurol. 467, 60–79 (2003)
Miyoshi, G., Butt, S. J., Takebayashi, H. & Fishell, G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J. Neurosci. 27, 7786–7798 (2007)
Acknowledgements
We are grateful to R. Batista-Brito, E. Chiappe, R. Cossart, J. Dasen, J. Kaltschmidt, S. Lee, J. Hjerling Leffler, M. Long, D. Pisapia and B. Rudy for comments on the manuscript. We thank L. Yin for technical assistance. We are indebted to K. Ravichandran for providing the ELMO1 constructs. N.V.D.G. and T.K. are both supported by grants from The Patterson Trust. Research in the Fishell laboratory is supported by the National Institutes of Health, National Institute of Mental Health (5RO1MH068469-08 and 2R01MH071679-09), National Institute of Neurological Disorders and Stroke (5R01NS039007-1), New York Stem Cell Science State (NGSG-130) and the Simons Foundation.
Author information
Authors and Affiliations
Contributions
N.V.D.G. and G.F. conceived the project. N.V.D.G. and T.K. designed and carried out the experiments. N.V.D.G. wrote the manuscript with the help of all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-10 with legends, a Supplementary Discussion, Supplementary Data and additional references. (PDF 823 kb)
Rights and permissions
About this article
Cite this article
De Marco García, N., Karayannis, T. & Fishell, G. Neuronal activity is required for the development of specific cortical interneuron subtypes. Nature 472, 351–355 (2011). https://doi.org/10.1038/nature09865
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09865
This article is cited by
-
Excitation–transcription coupling, neuronal gene expression and synaptic plasticity
Nature Reviews Neuroscience (2023)
-
Prominent in vivo influence of single interneurons in the developing barrel cortex
Nature Neuroscience (2023)
-
A Spacetime Odyssey of Neural Progenitors to Generate Neuronal Diversity
Neuroscience Bulletin (2023)
-
Step by step: cells with multiple functions in cortical circuit assembly
Nature Reviews Neuroscience (2022)
-
Perineuronal nets are under the control of type-5 metabotropic glutamate receptors in the developing somatosensory cortex
Translational Psychiatry (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.