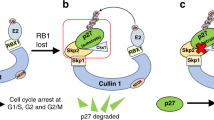
Abstract
The effective use of targeted therapy is highly dependent on the identification of responder patient populations. Loss of FBW7, which encodes a tumour-suppressor protein, is frequently found in various types of human cancer, including breast cancer, colon cancer1 and T-cell acute lymphoblastic leukaemia (T-ALL)2. In line with these genomic data, engineered deletion of Fbw7 in mouse T cells results in T-ALL3,4,5, validating FBW7 as a T-ALL tumour suppressor. Determining the precise molecular mechanisms by which FBW7 exerts antitumour activity is an area of intensive investigation. These mechanisms are thought to relate in part to FBW7-mediated destruction of key proteins relevant to cancer, including Jun6, Myc7, cyclin E8 and notch 1 (ref. 9), all of which have oncoprotein activity and are overexpressed in various human cancers, including leukaemia. In addition to accelerating cell growth10, overexpression of Jun, Myc or notch 1 can also induce programmed cell death11. Thus, considerable uncertainty surrounds how FBW7-deficient cells evade cell death in the setting of upregulated Jun, Myc and/or notch 1. Here we show that the E3 ubiquitin ligase SCFFBW7 (a SKP1–cullin-1–F-box complex that contains FBW7 as the F-box protein) governs cellular apoptosis by targeting MCL1, a pro-survival BCL2 family member, for ubiquitylation and destruction in a manner that depends on phosphorylation by glycogen synthase kinase 3. Human T-ALL cell lines showed a close relationship between FBW7 loss and MCL1 overexpression. Correspondingly, T-ALL cell lines with defective FBW7 are particularly sensitive to the multi-kinase inhibitor sorafenib but resistant to the BCL2 antagonist ABT-737. On the genetic level, FBW7 reconstitution or MCL1 depletion restores sensitivity to ABT-737, establishing MCL1 as a therapeutically relevant bypass survival mechanism that enables FBW7-deficient cells to evade apoptosis. Therefore, our work provides insight into the molecular mechanism of direct tumour suppression by FBW7 and has implications for the targeted treatment of patients with FBW7-deficient T-ALL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wood, L. D. et al. The genomic landscapes of human breast and colorectal cancers. Science 318, 1108–1113 (2007)
Maser, R. S. et al. Chromosomally unstable mouse tumours have genomic alterations similar to diverse human cancers. Nature 447, 966–971 (2007)
Onoyama, I. et al. Conditional inactivation of Fbxw7 impairs cell-cycle exit during T cell differentiation and results in lymphomatogenesis. J. Exp. Med. 204, 2875–2888 (2007)
Matsuoka, S. et al. Fbxw7 acts as a critical fail-safe against premature loss of hematopoietic stem cells and development of T-ALL. Genes Dev. 22, 986–991 (2008)
Thompson, B. J. et al. The SCFFBW7 ubiquitin ligase complex as a tumor suppressor in T cell leukemia. J. Exp. Med. 204, 1825–1835 (2007)
Wei, W., Jin, J., Schlisio, S., Harper, J. W. & Kaelin, W. G., Jr The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell 8, 25–33 (2005)
Welcker, M. et al. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc. Natl Acad. Sci. USA 101, 9085–9090 (2004)
Koepp, D. M. et al. Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science 294, 173–177 (2001)
Gupta-Rossi, N. et al. Functional interaction between SEL-10, an F-box protein, and the nuclear form of activated Notch1 receptor. J. Biol. Chem. 276, 34371–34378 (2001)
Shaulian, E. & Karin, M. AP-1 as a regulator of cell life and death. Nature Cell Biol. 4, E131–E136 (2002)
Sanchez, I. & Yuan, J. A convoluted way to die. Neuron 29, 563–566 (2001)
Akgul, C. Mcl-1 is a potential therapeutic target in multiple types of cancer. Cell. Mol. Life Sci. 66, 1326–1336 (2009)
Maurer, U., Charvet, C., Wagman, A. S., Dejardin, E. & Green, D. R. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol. Cell 21, 749–760 (2006)
Opferman, J. T. et al. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 426, 671–676 (2003)
Wertz, I. E. et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature doi:10.1038/nature09779 (this issue).
Welcker, M. & Clurman, B. E. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nature Rev. Cancer 8, 83–93 (2008)
Ding, Q. et al. Degradation of Mcl-1 by β-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Mol. Cell. Biol. 27, 4006–4017 (2007)
Nijhawan, D. et al. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 17, 1475–1486 (2003)
Panka, D. J., Cho, D. C., Atkins, M. B. & Mier, J. W. GSK-3β inhibition enhances sorafenib-induced apoptosis in melanoma cell lines. J. Biol. Chem. 283, 726–732 (2008)
Yu, C. et al. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene 24, 6861–6869 (2005)
Sharma, S. V. & Settleman, J. Oncogene addiction: setting the stage for molecularly targeted cancer therapy. Genes Dev. 21, 3214–3231 (2007)
Cragg, M. S., Harris, C., Strasser, A. & Scott, C. L. Unleashing the power of inhibitors of oncogenic kinases through BH3 mimetics. Nature Rev. Cancer 9, 321–326 (2009)
Konopleva, M. et al. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax). Cancer Res. 68, 3413–3420 (2008)
van Delft, M. F. et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 10, 389–399 (2006)
Zhong, Q., Gao, W., Du, F. & Wang, X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell 121, 1085–1095 (2005)
Schwickart, M. et al. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature 463, 103–107 (2010)
Popov, N. et al. The ubiquitin-specific protease USP28 is required for MYC stability. Nature Cell Biol. 9, 765–774 (2007)
Gao, D. et al. Phosphorylation by Akt1 promotes cytoplasmic localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction. Nature Cell Biol. 11, 397–408 (2009)
Benmaamar, R. & Pagano, M. Involvement of the SCF complex in the control of Cdh1 degradation in S-phase. Cell Cycle 4, 1230–1232 (2005)
Chen, D. et al. ARF-BP1/Mule is a critical mediator of the ARF tumor suppressor. Cell 121, 1071–1083 (2005)
Acknowledgements
We thank J. Lawler, C. Schorl, Q. Zhang and S. Glueck for critical reading of the manuscript, J. DeCaprio, M.-C. Hung, M. A. Kelliher, W. Harper and W. Hahn for providing reagents, L. Cantley and A. Toker for suggestions, I. Wertz and V. Dixit for sharing unpublished data, and members of the Wei and DePinho labs for useful discussions. W.W. is a Kimmel Scholar and V Scholar. This work was supported in part by the Emerald Foundation New Investigator award (W.W.), the Leukemia and Lymphoma Society Special Fellow award (W.W.) and a grant from the National Institutes of Health (W.W.; GM089763). R.A.D. is an American Cancer Society Research Professor and is supported by the Robert A. and Renée E. Belfer Foundation Institute for Applied Cancer Science.
Author information
Authors and Affiliations
Contributions
H.I. performed most of the experiments with critical assistance from S.S. and D.G. A.T., L.W. and A.W.L. also helped perform a portion of the experiments. I.O. performed the Fbw7 conditional knockout mouse experiments, and A.L.C. performed the orthotopic engraftment mouse experiments. B.Z. performed the mass spectrometry analysis, and Y.X. and R.S.M. helped to perform the experiments with tumours derived from the TKO mice. A.G. helped to perform the experiments with the human T-ALL clinical samples. W.W., R.A.D. and K.I.N. designed the experiments with assistance from J.A., J. S., A.L.K., H.I., S.P.G. and T.L. W.W. supervised the study. W.W. wrote the manuscript with help from H.I. and S.S. All authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
The file contains Supplementary Figures 1-11 with legends. (PDF 3510 kb)
Rights and permissions
About this article
Cite this article
Inuzuka, H., Shaik, S., Onoyama, I. et al. SCFFBW7 regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature 471, 104–109 (2011). https://doi.org/10.1038/nature09732
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09732
This article is cited by
-
FBXW7 regulates the sensitivity of imatinib in gastrointestinal stromal tumors by targeting MCL1
Gastric Cancer (2024)
-
O-GlcNAcylation of YTHDF2 promotes HBV-related hepatocellular carcinoma progression in an N6-methyladenosine-dependent manner
Signal Transduction and Targeted Therapy (2023)
-
FBXW7β loss-of-function enhances FASN-mediated lipogenesis and promotes colorectal cancer growth
Signal Transduction and Targeted Therapy (2023)
-
FBXW7 tumor suppressor regulation by dualspecificity tyrosine-regulated kinase 2
Cell Death & Disease (2023)
-
Transient targeting of BIM-dependent adaptive MCL1 preservation enhances tumor response to molecular therapeutics in non-small cell lung cancer
Cell Death & Differentiation (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.