Abstract
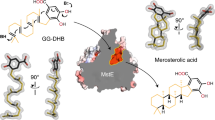
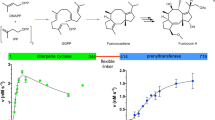
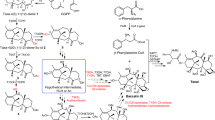
With more than 55,000 members identified so far in all forms of life, the family of terpene or terpenoid natural products represents the epitome of molecular biodiversity. A well-known and important member of this family is the polycyclic diterpenoid Taxol (paclitaxel), which promotes tubulin polymerization1 and shows remarkable efficacy in cancer chemotherapy2. The first committed step of Taxol biosynthesis in the Pacific yew (Taxus brevifolia)3 is the cyclization of the linear isoprenoid substrate geranylgeranyl diphosphate (GGPP) to form taxa-4(5),11(12)diene4, which is catalysed by taxadiene synthase5. The full-length form of this diterpene cyclase contains 862 residues, but a roughly 80-residue amino-terminal transit sequence is cleaved on maturation in plastids6. We now report the X-ray crystal structure of a truncation variant lacking the transit sequence and an additional 27 residues at the N terminus, hereafter designated TXS. Specifically, we have determined structures of TXS complexed with 13-aza-13,14-dihydrocopalyl diphosphate (1.82 Å resolution) and 2-fluorogeranylgeranyl diphosphate (2.25 Å resolution). The TXS structure reveals a modular assembly of three α-helical domains. The carboxy-terminal catalytic domain is a class I terpenoid cyclase, which binds and activates substrate GGPP with a three-metal ion cluster. The N-terminal domain and a third ‘insertion’ domain together adopt the fold of a vestigial class II terpenoid cyclase. A class II cyclase activates the isoprenoid substrate by protonation instead of ionization, and the TXS structure reveals a definitive connection between the two distinct cyclase classes in the evolution of terpenoid biosynthesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schiff, P. B., Fant, J. & Horwitz, S. B. Promotion of microtubule assembly in vitro by taxol. Nature 277, 665–667 (1979)
Arbuck, S. G. & Blaylock, B. A. in Taxol: Science and Applications (ed. Suffness, M.) 379–415 (CRC Press, 1995)
Wani, M. C., Taylor, H. L., Wall, M., Coggon, P. & McPhail, A. T. Plant antitumor agents. VI. The isolation and structure of Taxol, a novel antileukemic and antitumor agent from Taxus brevifolia . J. Am. Chem. Soc. 93, 2325–2327 (1971)
Koepp, A. E. et al. Cyclization of geranylgeranyl diphosphate to taxa-4(5),11(12)-diene is the committed step of Taxol biosynthesis in Pacific yew. J. Biol. Chem. 270, 8686–8690 (1995)
Hezari, M., Lewis, N. G. & Croteau, R. Purification and characterization of taxa-4(5),11(12)-diene synthase from Pacific yew (Taxus brevifolia) that catalyzes the first committed step of Taxol biosynthesis. Arch. Biochem. Biophys. 322, 437–444 (1995)
Wildung, M. R. & Croteau, R. A cDNA clone for taxadiene synthase, the diterpene cyclase that catalyzes the committed step of Taxol biosynthesis. J. Biol. Chem. 271, 9201–9204 (1996)
Whittington, D. A. et al. Bornyl diphosphate synthase: structure and strategy for carbocation manipulation by a terpenoid cyclase. Proc. Natl Acad. Sci. USA 99, 15375–15380 (2002)
Lesburg, C. A., Zhai, G., Cane, D. E. & Christianson, D. W. Crystal structure of pentalenene synthase: mechanistic insights on terpenoid cyclization reactions in biology. Science 277, 1820–1824 (1997)
Starks, C. M., Back, K., Chappell, J. & Noel, J. P. Structural basis for cyclic terpene biosynthesis by tobacco 5-epi-aristolochene synthase. Science 277, 1815–1820 (1997)
Wendt, K. U., Poralla, K. & Schulz, G. E. Structure and function of a squalene cyclase. Science 277, 1811–1815 (1997)
Trapp, S. C. & Croteau, R. Genomic organization of plant terpene synthases and molecular evolutionary implications. Genetics 158, 811–832 (2001)
Keeling, C. I. et al. Identification and functional characterization of monofunctional ent-copalyl diphosphate and ent-kaurene synthases in white spruce reveal different patterns for diterpene synthase evolution for primary and secondary metabolism in gymnosperms. Plant Physiol. 152, 1197–1208 (2010)
Cao, R. et al. Diterpene cyclases and the nature of the isoprene fold. Proteins Struct. Funct. Bioinf. 78, 2417–2432 (2010)
Wendt, K. U. & Schulz, G. E. Isoprenoid biosynthesis: manifold chemistry catalyzed by similar enzymes. Structure 6, 127–133 (1998)
Christianson, D. W. Structural biology and chemistry of the terpenoid cyclases. Chem. Rev. 106, 3412–3442 (2006)
Christianson, D. W. Unearthing the roots of the terpenome. Curr. Opin. Chem. Biol. 12, 141–150 (2008)
Wendt, K. U., Schulz, G. E., Corey, E. J. & Liu, D. R. Enzyme mechanisms for polycyclic triterpene formation. Angew. Chem. Int. Ed. 39, 2812–2833 (2000)
Lin, X., Hezari, M., Koepp, A. E., Floss, H. G. & Croteau, R. Mechanism of taxadiene synthase, a diterpene cyclase that catalyzes the first step of Taxol biosynthesis in Pacific yew. Biochemistry 35, 2968–2977 (1996)
Williams, D. C. et al. Heterologous expression and characterization of a ‘pseudomature’ form of taxadiene synthase involved in paclitaxel (Taxol) biosynthesis and evaluation of a potential intermediate and inhibitors of the multistep diterpene cyclization reaction. Arch. Biochem. Biophys. 379, 137–146 (2000)
Williams, D. C. et al. Intramolecular proton transfer in the cyclization of geranylgeranyl diphosphate to the taxadiene precursor of taxol catalyzed by recombinant taxadiene synthase. Chem. Biol. 7, 969–977 (2000)
Jin, Q., Williams, D. C., Hezari, M., Croteau, R. & Coates, R. M. Stereochemistry of the macrocyclization and elimination steps in taxadiene biosynthesis through deuterium labelling. J. Org. Chem. 70, 4667–4675 (2005)
Jin, Y., Williams, D. C., Croteau, R. & Coates, R. M. Taxadiene synthase-catalyzed cyclization of 6-fluorogeranylgeranyl diphosphate to 7-fluoroverticillenes. J. Am. Chem. Soc. 127, 7834–7842 (2005)
Tarshis, L. C., Yan, M., Poulter, C. D. & Sacchettini, J. C. Crystal structure of recombinant farnesyl diphosphate synthase at 2.6-Å resolution. Biochemistry 33, 10871–10877 (1994)
Chang, T. H., Guo, R. T., Ko, T. P., Wang, A. H. J. & Liang, P. H. Crystal structure of type-III geranylgeranyl pyrophosphate synthase from Saccharomyces cerevisiae and the mechanism of product chain length determination. J. Biol. Chem. 281, 14991–15000 (2006)
Bohlmann, J., Meyer-Gauen, G. & Croteau, R. Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc. Natl Acad. Sci. USA 95, 4126–4133 (1998)
Thoma, R. et al. Insight into steroid scaffold formation from the structure of human oxidosqualene cyclase. Nature 432, 118–122 (2004)
Rynkiewicz, M. J., Cane, D. E. & Christianson, D. W. Structure of trichodiene synthase from Fusarium sporotrichioides provides mechanistic inferences on the terpene cyclization cascade. Proc. Natl Acad. Sci. USA 98, 13543–13548 (2001)
Köksal, M., Zimmer, I., Schnitzler, J.-P. & Christianson, D. W. Structure of isoprene synthase illuminates the chemical mechanism of teragram atmospheric carbon emission. J. Mol. Biol. 402, 363–373 (2010)
Peters, R. J., Ravn, M. M., Coates, R. M. & Croteau, R. Bifunctional abietadiene synthase: free diffusive transfer of the (+)-copalyl diphosphate intermediate between two distinct active sites. J. Am. Chem. Soc. 123, 8974–8978 (2001)
Prisic, S. & Peters, R. J. Synergistic substrate inhibition of ent-copalyl diphosphate synthase: a potential feed-forward inhibition mechanism limiting gibberellin metabolism. Plant Physiol. 144, 445–454 (2007)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
Pape, T. & Schneider, T. R. HKL2MAP: a graphical user interface for phasing with SHELX programs. J. Appl. Cryst. 37, 843–844 (2004)
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D66, 213–221 (2010)
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D66, 486–501 (2010)
Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. PROCHECK—a program to check the stereochemical quality of protein structures. J. Appl. Cryst. 26, 283–291 (1993)
Brünger, A. T. et al. Crystallography & NMR System: a new software suite for macromolecular structure determination. Acta Crystallogr. D54, 905–921 (1998)
Phillips, J. C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005)
MacKerell, A. D., Jr et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 102, 3586–3616 (1998)
Kleywegt, G. J. & Jones, T. A. Detection, delineation, measurement and display of cavities in macromolecular structures. Acta Crystallogr. D50, 178–185 (1994)
Gao, Q. & Chen, S. H. An unprecedented side chain conformation of paclitaxel (Taxol®): crystal structure of 7-mesylpaclitaxel. Tetrahedr. Lett. 37, 3425–3428 (1996)
Acknowledgements
We thank C. MacDermaid and J. Saven for advice and assistance with molecular modelling calculations, and E. Oldfield for helpful comments on the manuscript. We thank the National Synchrotron Light Source at Brookhaven National Laboratory for beamline access. The US National Institutes of Health provided grants GM56838 (D.W.C.), GM13956 (R.M.C.) and CA55254 (R.C.) in support of this research. Y.J. thanks the University of Illinois for support through the John C. Bailar and R. C. Fuson Fellowships.
Author information
Authors and Affiliations
Contributions
M.K. and D.W.C. performed the X-ray crystallographic studies. R.C. supplied the M79-TXS construct from which M107-TXS-CHT was ultimately prepared. Y.J. and R.M.C. synthesized 2-fluorogeranylgeranyl diphosphate. All authors contributed to the interpretation of the results and preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, additional references, Supplementary Tables 1-2 and Supplementary Figures 1-4 with legends. (PDF 4513 kb)
Rights and permissions
About this article
Cite this article
Köksal, M., Jin, Y., Coates, R. et al. Taxadiene synthase structure and evolution of modular architecture in terpene biosynthesis. Nature 469, 116–120 (2011). https://doi.org/10.1038/nature09628
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09628
This article is cited by
-
Ancient plant-like terpene biosynthesis in corals
Nature Chemical Biology (2022)
-
Biosynthesis of paclitaxel using synthetic biology
Phytochemistry Reviews (2022)
-
An Overview on Taxol Production Technology and Its Applications as Anticancer Agent
Biotechnology and Bioprocess Engineering (2022)
-
Mining methods and typical structural mechanisms of terpene cyclases
Bioresources and Bioprocessing (2021)
-
Kinetic studies and homology modeling of a dual-substrate linalool/nerolidol synthase from Plectranthus amboinicus
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.