Abstract
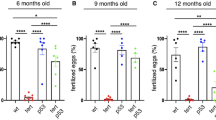
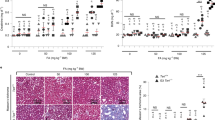
An ageing world population has fuelled interest in regenerative remedies that may stem declining organ function and maintain fitness. Unanswered is whether elimination of intrinsic instigators driving age-associated degeneration can reverse, as opposed to simply arrest, various afflictions of the aged. Such instigators include progressively damaged genomes. Telomerase-deficient mice have served as a model system to study the adverse cellular and organismal consequences of wide-spread endogenous DNA damage signalling activation in vivo1. Telomere loss and uncapping provokes progressive tissue atrophy, stem cell depletion, organ system failure and impaired tissue injury responses1. Here, we sought to determine whether entrenched multi-system degeneration in adult mice with severe telomere dysfunction can be halted or possibly reversed by reactivation of endogenous telomerase activity. To this end, we engineered a knock-in allele encoding a 4-hydroxytamoxifen (4-OHT)-inducible telomerase reverse transcriptase-oestrogen receptor (TERT-ER) under transcriptional control of the endogenous TERT promoter. Homozygous TERT-ER mice have short dysfunctional telomeres and sustain increased DNA damage signalling and classical degenerative phenotypes upon successive generational matings and advancing age. Telomerase reactivation in such late generation TERT-ER mice extends telomeres, reduces DNA damage signalling and associated cellular checkpoint responses, allows resumption of proliferation in quiescent cultures, and eliminates degenerative phenotypes across multiple organs including testes, spleens and intestines. Notably, somatic telomerase reactivation reversed neurodegeneration with restoration of proliferating Sox2+ neural progenitors, Dcx+ newborn neurons, and Olig2+ oligodendrocyte populations. Consistent with the integral role of subventricular zone neural progenitors in generation and maintenance of olfactory bulb interneurons2, this wave of telomerase-dependent neurogenesis resulted in alleviation of hyposmia and recovery of innate olfactory avoidance responses. Accumulating evidence implicating telomere damage as a driver of age-associated organ decline and disease risk1,3 and the marked reversal of systemic degenerative phenotypes in adult mice observed here support the development of regenerative strategies designed to restore telomere integrity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sahin, E. & Depinho, R. A. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 464, 520–528 (2010)
Whitman, M. C. & Greer, C. A. Adult neurogenesis and the olfactory system. Prog. Neurobiol. 89, 162–175 (2009)
Sharpless, N. E. & DePinho, R. A. How stem cells age and why this makes us grow old. Nature Rev. Mol. Cell Biol. 8, 703–713 (2007)
Ju, Z. & Lenhard Rudolph, K. Telomere dysfunction and stem cell ageing. Biochimie 90, 24–32 (2008)
Hoeijmakers, J. H. DNA damage, aging, and cancer. N. Engl. J. Med. 361, 1475–1485 (2009)
Ferrón, S. et al. Telomere shortening and chromosomal instability abrogates proliferation of adult but not embryonic neural stem cells. Development 131, 4059–4070 (2004)
Cawthon, R. M. et al. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361, 393–395 (2003)
Wyllie, F. S. et al. Telomerase prevents the accelerated cell ageing of Werner syndrome fibroblasts. Nature Genet. 24, 16–17 (2000)
Bodnar, A. G. et al. Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352 (1998)
Tomás-Loba, A. et al. Telomerase reverse transcriptase delays aging in cancer-resistant mice. Cell 135, 609–622 (2008)
Samper, E., Flores, J. M. & Blasco, M. A. Restoration of telomerase activity rescues chromosomal instability and premature aging in Terc−/− mice with short telomeres. EMBO Rep. 2, 800–807 (2001)
Metzger, D. et al. Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proc. Natl Acad. Sci. USA 92, 6991–6995 (1995)
Blasco, M. A. et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91, 25–34 (1997)
Wong, K. K. et al. Telomere dysfunction and Atm deficiency compromises organ homeostasis and accelerates ageing. Nature 421, 643–648 (2003)
Choudhury, A. R. et al. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nature Genet. 39, 99–105 (2006)
Best, B. P. Nuclear DNA damage as a direct cause of aging. Rejuvenation Res. 12, 199–208 (2009)
Drapeau, E. & Nora Abrous, D. Stem cell review series: role of neurogenesis in age-related memory disorders. Aging Cell 7, 569–589 (2008)
Ferrón, S. R. et al. Telomere shortening in neural stem cells disrupts neuronal differentiation and neuritogenesis. J. Neurosci. 29, 14394–14407 (2009)
Enwere, E. et al. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J. Neurosci. 24, 8354–8365 (2004)
Lafreniere, D. & Mann, N. Anosmia: loss of smell in the elderly. Otolaryngol. Clin. North Am. 42, 123–131 (2009)
Ma, D. K. et al. Activity-dependent extrinsic regulation of adult olfactory bulb and hippocampal neurogenesis. Ann. NY Acad. Sci. 1170, 664–673 (2009)
Kobayakawa, K. et al. Innate versus learned odour processing in the mouse olfactory bulb. Nature 450, 503–508 (2007)
Caporaso, G. L. et al. Telomerase activity in the subventricular zone of adult mice. Mol. Cell. Neurosci. 23, 693–702 (2003)
Artandi, S. E. & DePinho, R. A. Telomeres and telomerase in cancer. Carcinogenesis 31, 9–18 (2010)
Zhang, P., Dilley, C. & Mattson, M. P. DNA damage responses in neural cells: focus on the telomere. Neuroscience 145, 1439–1448 (2007)
Lee, J. et al. Telomerase deficiency affects normal brain functions in mice. Neurochem. Res. 35, 211–218 (2010)
Breton-Provencher, V. et al. Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. J. Neurosci. 29, 15245–15257 (2009)
Maser, R. S. et al. DNA-dependent protein kinase catalytic subunit is not required for dysfunctional telomere fusion and checkpoint response in the telomerase-deficient mouse. Mol. Cell. Biol. 27, 2253–2265 (2007)
Abramoff, M. D., Magelhaes, P. J. & Ram, S. J. Image Processing with ImageJ. Biophotonics Int. 11, 36–42 (2001)
Potzner, M. R. et al. Prolonged Sox4 expression in oligodendrocytes interferes with normal myelination in the central nervous system. Mol. Cell. Biol. 27, 5316–5326 (2007)
Shao, C. et al. Mitotic recombination produces the majority of recessive fibroblast variants in heterozygous mice. Proc. Natl Acad. Sci. USA 96, 9230–9235 (1999)
Paik, J. H. et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell 5, 540–553 (2009)
Mahoney, J. E. et al. Quantification of telomere length by FISH and laser scanning cytometry. Proc. SPIE 6859, 1–9 (2008)
Gorczyca, W. et al. Analysis of human tumors by laser scanning cytometry. Methods Cell Biol. 64, 421–443 (2001)
Spink, A. J. et al. The EthoVision video tracking system–a tool for behavioral phenotyping of transgenic mice. Physiol. Behav. 73, 731–744 (2001)
Acknowledgements
The authors would like to thank R. Segal for critical comments, R. Bronson, K. Ligon and C. Maire for histological advice, S. S. Chae for assistance with neurosphere measurement studies and L. Cameron for time-lapse microscopy studies. M.J. was supported in part by a Susan G. Komen for the Cure fellowship (PDF060881). F.L.M. was supported by ACS fellowship PF-08-261-01-TBE. This work and R.A.D. was supported by R01CA84628 and U01CA141508 grants from the NIH National Cancer Institute and the Belfer Foundation. R.A.D. was supported by an American Cancer Society Research Professorship.
Author information
Authors and Affiliations
Contributions
M.J. and R.A.D. designed and guided the research; M.J., F.L.M., J.-H.P., E.S., E.T., S.J. and M.K.-A. performed research. J.C. and J.W.H. generated the TERT-ER mouse. M.J., F.L.M., A.C.A., A.P., E.M.-F. and R.A.D. analysed data. M.J. and R.A.D. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-4 with legends and Supplementary Tables 1-3. (PDF 751 kb)
Rights and permissions
About this article
Cite this article
Jaskelioff, M., Muller, F., Paik, JH. et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 469, 102–106 (2011). https://doi.org/10.1038/nature09603
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09603
This article is cited by
-
Chromosome ends and the theory of marginotomy: implications for reproduction
Biogerontology (2024)
-
Senescence program and its reprogramming in pancreatic premalignancy
Cell Death & Disease (2023)
-
Boosting NAD ameliorates hematopoietic impairment linked to short telomeres in vivo
GeroScience (2023)
-
HMGB1 is a Potential and Challenging Therapeutic Target for Parkinson’s Disease
Cellular and Molecular Neurobiology (2023)
-
Is telomerase a hidden player? Therapeutic potential of natural telomerase activators against age-related diseases
Phytochemistry Reviews (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.