Abstract
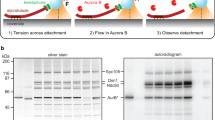
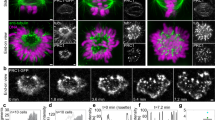
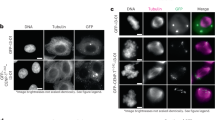
Kinetochores are macromolecular machines that couple chromosomes to dynamic microtubule tips during cell division, thereby generating force to segregate the chromosomes1,2. Accurate segregation depends on selective stabilization of correct ‘bi-oriented’ kinetochore–microtubule attachments, which come under tension as the result of opposing forces exerted by microtubules3. Tension is thought to stabilize these bi-oriented attachments indirectly, by suppressing the destabilizing activity of a kinase, Aurora B4,5. However, a complete mechanistic understanding of the role of tension requires reconstitution of kinetochore–microtubule attachments for biochemical and biophysical analyses in vitro. Here we show that native kinetochore particles retaining the majority of kinetochore proteins can be purified from budding yeast and used to reconstitute dynamic microtubule attachments. Individual kinetochore particles maintain load-bearing associations with assembling and disassembling ends of single microtubules for >30 min, providing a close match to the persistent coupling seen in vivo between budding yeast kinetochores and single microtubules6. Moreover, tension increases the lifetimes of the reconstituted attachments directly, through a catch bond-like mechanism that does not require Aurora B7,8,9,10. On the basis of these findings, we propose that tension selectively stabilizes proper kinetochore–microtubule attachments in vivo through a combination of direct mechanical stabilization and tension-dependent phosphoregulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Santaguida, S. & Musacchio, A. The life and miracles of kinetochores. EMBO J. 28, 2511–2531 (2009)
Cheeseman, I. M. & Desai, A. Molecular architecture of the kinetochore-microtubule interface. Nature Rev. Mol. Cell Biol. 9, 33–46 (2008)
Nicklas, R. B. The forces that move chromosomes in mitosis. Annu. Rev. Biophys. Biophys. Chem. 17, 431–449 (1988)
Maresca, T. J. & Salmon, E. D. Welcome to a new kind of tension: translating kinetochore mechanics into a wait-anaphase signal. J. Cell Sci. 123, 825–835 (2010)
Liu, D., Vader, G., Vromans, M. J., Lampson, M. A. & Lens, S. M. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science 323, 1350–1353 (2009)
Straight, A. F., Marshall, W. F., Sedat, J. W. & Murray, A. W. Mitosis in living budding yeast: anaphase A but no metaphase plate. Science 277, 574–578 (1997)
McEver, R. P. & Zhu, C. Rolling cell adhesion. Annu. Rev. Cell Dev. Biol. 26, 363–396 (2010)
Sarangapani, K. K. et al. Low force decelerates L-selectin dissociation from P-selectin glycoprotein ligand-1 and endoglycan. J. Biol. Chem. 279, 2291–2298 (2004)
Thomas, W. E., Vogel, V. & Sokurenko, E. Biophysics of catch bonds. Annu Rev Biophys 37, 399–416 (2008)
Marshall, B. T. et al. Direct observation of catch bonds involving cell-adhesion molecules. Nature 423, 190–193 (2003)
Akiyoshi, B., Nelson, C. R., Ranish, J. A. & Biggins, S. Quantitative proteomic analysis of purified yeast kinetochores identifies a PP1 regulatory subunit. Genes Dev. 23, 2887–2899 (2009)
De Wulf, P., McAinsh, A. D. & Sorger, P. K. Hierarchical assembly of the budding yeast kinetochore from multiple subcomplexes. Genes Dev. 17, 2902–2921 (2003)
Nekrasov, V. S., Smith, M. A., Peak-Chew, S. & Kilmartin, J. V. Interactions between centromere complexes in Saccharomyces cerevisiae . Mol. Biol. Cell 14, 4931–4946 (2003)
Cheeseman, I. M., Chappie, J. S., Wilson-Kubalek, E. M. & Desai, A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127, 983–997 (2006)
Tanaka, K. et al. Molecular mechanisms of kinetochore capture by spindle microtubules. Nature 434, 987–994 (2005)
Powers, A. F. et al. The Ndc80 kinetochore complex forms load-bearing attachments to dynamic microtubule tips via biased diffusion. Cell 136, 865–875 (2009)
Franck, A. D. et al. Tension applied through the Dam1 complex promotes microtubule elongation providing a direct mechanism for length control in mitosis. Nature Cell Biol. 9, 832–837 (2007)
Tien, J. F. et al. Cooperation of the Dam1 and Ndc80 kinetochore complexes enhances microtubule coupling and is regulated by aurora B. J. Cell Biol. 189, 713–723 (2010)
Franck, A. D., Powers, A. F., Gestaut, D. R., Davis, T. N. & Asbury, C. L. Direct physical study of kinetochore-microtubule interactions by reconstitution and interrogation with an optical force clamp. Methods 51, 242–250 (2010)
Howard, J., Hudspeth, A. J. & Vale, R. D. Movement of microtubules by single kinesin molecules. Nature 342, 154–158 (1989)
Block, S. M., Goldstein, L. S. & Schnapp, B. J. Bead movement by single kinesin molecules studied with optical tweezers. Nature 348, 348–352 (1990)
Mehta, A. D. et al. Myosin-V is a processive actin-based motor. Nature 400, 590–593 (1999)
Winey, M. et al. Three-dimensional ultrastructural analysis of the Saccharomyces cerevisiae mitotic spindle. J. Cell Biol. 129, 1601–1615 (1995)
Nicklas, R. B. & Ward, S. C. Elements of error correction in mitosis: microtubule capture, release, and tension. J. Cell Biol. 126, 1241–1253 (1994)
Bell, G. I. Models for the specific adhesion of cells to cells. Science 200, 618–627 (1978)
Merkel, R., Nassoy, P., Leung, A., Ritchie, K. & Evans, E. Energy landscapes of receptor-ligand bonds explored with dynamic force spectroscopy. Nature 397, 50–53 (1999)
Rose, M. D., Winston, F. & Heiter, P. Methods in yeast genetics. (Cold Spring Harbor Laboratory Press, 1990)
Biggins, S. et al. The conserved protein kinase Ipl1 regulates microtubule binding to kinetochores in budding yeast. Genes Dev. 13, 532–544 (1999)
Wigge, P. A. et al. Analysis of the Saccharomyces spindle pole by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry. J. Cell Biol. 141, 967–977 (1998)
Enquist-Newman, M. et al. Dad1p, third component of the Duo1p/Dam1p complex involved in kinetochore function and mitotic spindle integrity. Mol. Biol. Cell 12, 2601–2613 (2001)
Longtine, M. S. et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae . Yeast 14, 953–961 (1998)
Gelbart, M. E., Rechsteiner, T., Richmond, T. J. & Tsukiyama, T. Interactions of Isw2 chromatin remodeling complex with nucleosomal arrays: analyses using recombinant yeast histones and immobilized templates. Mol. Cell. Biol. 21, 2098–2106 (2001)
Sikorski, R. S. & Hieter, P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae . Genetics 122, 19–27 (1989)
Pinsky, B. A., Tatsutani, S. Y., Collins, K. A. & Biggins, S. An Mtw1 complex promotes kinetochore biorientation that is monitored by the Ipl1/Aurora protein kinase. Dev. Cell 5, 735–745 (2003)
Buvelot, S., Tatsutani, S. Y., Vermaak, D. & Biggins, S. The budding yeast Ipl1/Aurora protein kinase regulates mitotic spindle disassembly. J. Cell Biol. 160, 329–339 (2003)
Kitamura, E., Tanaka, K., Kitamura, Y. & Tanaka, T. U. Kinetochore microtubule interaction during S phase in Saccharomyces cerevisiae . Genes Dev. 21, 3319–3330 (2007)
Gestaut, D. R., Cooper, J., Asbury, C. L., Davis, T. N. & Wordeman, L. Reconstitution and functional analysis of kinetochore subcomplexes. Methods Cell Biol. 95, 641–656 (2010)
Rice, S. et al. A structural change in the kinesin motor protein that drives motility. Nature 402, 778–784 (1999)
Asbury, C. L., Gestaut, D. R., Powers, A. F., Franck, A. D. & Davis, T. N. The Dam1 kinetochore complex harnesses microtubule dynamics to produce force and movement. Proc. Natl Acad. Sci. USA 103, 9873–9878 (2006)
Acknowledgements
We thank A. Desai for antibodies, and J. Kilmartin, G. Barnes, D. Pellman, R. Tsien and the Yeast Resource Center for strains and plasmids. We also thank M. Press for constructing the Cse4–GFP strain, M. Yuan at the ISB for help, and the Seattle Mitosis Club for comments. We are grateful to T. Davis, W. Thomas, B. Zagotta, T. Tsukiyama, J. Stumpff, F. Rieke, S. Gordon and the Biggins lab for comments on the manuscript. This work was supported by an NSF IGERT fellowship (DGE-0504573) and NIH traineeship (T32GM07270) to A.F.P., an NIH Cardiovascular Pathology traineeship (T32HL007312) to K.K.S., a Beckman Young Investigator grant to S.B., NIH grants (GM078069 and GM064386) to S.B., an NCI Cancer Center Support grant (CA015704) and an NIGMS grant (PM50 GM076547/Center for Systems Biology) to J.A.R., a Searle Scholar Award (06-L-111) to C.L.A., a Packard Fellowship for Science and Engineering (2006-30521) to C.L.A. and an NIGMS grant (R01GM79373) to C.L.A. S.B. is a Scholar of the Leukemia and Lymphoma Society and T.G. is a Howard Hughes Medical Institute Early Career Scientist.
Author information
Authors and Affiliations
Contributions
All authors designed various components of the research. B.A. and C.R.N. constructed plasmids and yeast strains and B.A. purified kinetochore particles and analysed composition. B.A. and J.A.R. performed mass spectrometry and data analysis. A.F.P., K.K.S., H.A.S. and C.L.A. performed microtubule experiments. S.L.R. performed gel filtration.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-11 with legends, Supplementary Notes 1-7, Supplementary Tables 2- 4 (see separate file for Supplementary Table 1) and Supplementary References. (PDF 2459 kb)
Supplementary Table 1
This table contains the Dsn1-FLAG MS list. (XLS 422 kb)
Supplementary Movie 1
The movie shows Cse4-GFP kinetochore particles track with depolymerizing microtubule tips. (AVI 4792 kb)
Supplementary Movie 2
The movie shows Nuf2-3GFP kinetochore particles track with shortening tips. (AVI 1568 kb)
Supplementary Movie 3
The movie shows purified kinetochore particles couple force to dynamic microtubules. (AVI 7665 kb)
Rights and permissions
About this article
Cite this article
Akiyoshi, B., Sarangapani, K., Powers, A. et al. Tension directly stabilizes reconstituted kinetochore-microtubule attachments. Nature 468, 576–579 (2010). https://doi.org/10.1038/nature09594
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09594
This article is cited by
-
Higher-order protein assembly controls kinetochore formation
Nature Cell Biology (2024)
-
Dicentric chromosomes are resolved through breakage and repair at their centromeres
Chromosoma (2024)
-
Probing stress-regulated ordering of the plant cortical microtubule array via a computational approach
BMC Plant Biology (2023)
-
Catch bond models may explain how force amplifies TCR signaling and antigen discrimination
Nature Communications (2023)
-
Mechanisms underlying spindle assembly and robustness
Nature Reviews Molecular Cell Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.