Abstract
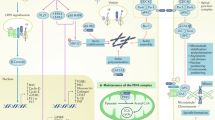
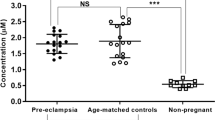
Blood pressure is critically controlled by angiotensins1, which are vasopressor peptides specifically released by the enzyme renin from the tail of angiotensinogen—a non-inhibitory member of the serpin family of protease inhibitors2,3. Although angiotensinogen has long been regarded as a passive substrate, the crystal structures solved here to 2.1 Å resolution show that the angiotensin cleavage site is inaccessibly buried in its amino-terminal tail. The conformational rearrangement that makes this site accessible for proteolysis is revealed in our 4.4 Å structure of the complex of human angiotensinogen with renin. The co-ordinated changes involved are seen to be critically linked by a conserved but labile disulphide bridge. Here we show that the reduced unbridged form of angiotensinogen is present in the circulation in a near 40:60 ratio with the oxidized sulphydryl-bridged form, which preferentially interacts with receptor-bound renin. We propose that this redox-responsive transition of angiotensinogen to a form that will more effectively release angiotensin at a cellular level contributes to the modulation of blood pressure. Specifically, we demonstrate the oxidative switch of angiotensinogen to its more active sulphydryl-bridged form in the maternal circulation in pre-eclampsia—the hypertensive crisis of pregnancy that threatens the health and survival of both mother and child.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Primary accessions
Protein Data Bank
Data deposits
Ethical approval was obtained for the use of all patient and control plasmas. Atomic coordinates and structure factors for the reported crystal structures have been deposited with the Protein Data Bank under accession codes 2WXX, 2WXY, 2WXZ, 2WXW, 2WY0, 2WY1 and 2X0B.
References
Fyhrquist, F. & Saijonmaa, O. Renin-angiotensin system revisited. J. Intern. Med. 264, 224–236 (2008)
Stein, P. E., Tewkesbury, D. A. & Carrell, R. W. Ovalbumin and angiotensinogen lack serpin S-R conformational change. Biochem. J. 262, 103–107 (1989)
Huber, R. & Carrell, R. W. Implications of the three-dimensional structure of α1-antitrypsin for structure and function of serpins. Biochemistry 28, 8951–8966 (1989)
Dickson, M. E. & Sigmund, C. D. Genetic basis of hypertension: revisiting angiotensinogen. Hypertension 48, 14–20 (2006)
Kim, H. S. et al. Genetic control of blood pressure and the angiotensinogen locus. Proc. Natl Acad. Sci. USA 92, 2735–2739 (1995)
Inoue, I. et al. A nucleotide substitution in the promoter of human angiotensinogen is associated with essential hypertension and affects basal transcription in vitro. J. Clin. Invest. 99, 1786–1797 (1997)
Dealwis, C. G. et al. X-ray analysis at 2.0 Å resolution of mouse submaxillary renin complexed with a decapeptide inhibitor CH-66, based on the 4–16 fragment of rat angiotensinogen. J. Mol. Biol. 236, 342–360 (1994)
Nasir, U. M. et al. Two peaks in pH dependence of renin-angiotensinogen reaction. Biosci. Biotechnol. Biochem. 62, 338–340 (1998)
Streatfeild-James, R. M. et al. Angiotensinogen cleavage by renin: importance of a structurally constrained N-terminus. FEBS Lett. 436, 267–270 (1998)
Gimenez-Roqueplo, A. P., Celerier, J., Schmid, G., Corvol, P. & Jeunemaitre, X. Role of cysteine residues in human angiotensinogen. Cys232 is required for angiotensinogen-pro major basic protein complex formation. J. Biol. Chem. 273, 34480–34487 (1998)
Kobori, H., Nangaku, M., Navar, L. G. & Nishiyama, A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol. Rev. 59, 251–287 (2007)
Nguyen, G. et al. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J. Clin. Invest. 109, 1417–1427 (2002)
Brandes, N., Schmitt, S. & Jakob, U. Thiol-based redox switches in eukaryotic proteins. Antioxid. Redox Signal. 11, 997–1014 (2009)
Myatt, L. Review: reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta 31 (suppl. A). S66–S69 (2010)
Dahm, C. C., Moore, K. & Murphy, M. P. Persistent S-nitrosation of complex I and other mitochondrial membrane proteins by S-nitrosothiols but not nitric oxide or peroxynitrite: implications for the interaction of nitric oxide with mitochondria. J. Biol. Chem. 281, 10056–10065 (2006)
Stamler, J. S., Lamas, S. & Fang, F. C. Nitrosylation: the prototypic redox-based signaling mechanism. Cell 106, 675–683 (2001)
Wilcox, C. S. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R913–R935 (2005)
Harrison, D. G. & Gongora, M. C. Oxidative stress and hypertension. Med. Clin. North Am. 93, 621–635 (2009)
Burton, G. J. & Jauniaux, E. Placental oxidative stress: from miscarriage to preeclampsia. J. Soc. Gynecol. Investig. 11, 342–352 (2004)
Hubel, C. A. Oxidative stress in the pathogenesis of preeclampsia. Proc. Soc. Exp. Biol. Med. 222, 222–235 (1999)
Sedeek, M. et al. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am. J. Hypertens. 21, 1152–1156 (2008)
Sibai, B., Dekker, G. & Kupferminc, M. Pre-eclampsia. Lancet 365, 785–799 (2005)
Mistry, H. D., Wilson, V., Ramsay, M. M., Symonds, M. E. & Broughton Pipkin, F. Reduced selenium concentrations and glutathione peroxidase activity in preeclamptic pregnancies. Hypertension 52, 881–888 (2008)
Bulgan Kilicdag, E. et al. Oxidant-antioxidant system changes relative to placental-umbilical pathology in patients with preeclampsia. Hypertens. Pregnancy 24, 147–157 (2005)
Inoue, I. et al. A mutation of angiotensinogen in a patient with preeclampsia leads to altered kinetics of the renin-angiotensin system. J. Biol. Chem. 270, 11430–11436 (1995)
Hoffmann, D. S. et al. Chronic tempol prevents hypertension, proteinuria, and poor feto-placental outcomes in BPH/5 mouse model of preeclampsia. Hypertension 51, 1058–1065 (2008)
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007)
Powell, N. A. et al. Benzyl ether structure-activity relationships in a series of ketopiperazine-based renin inhibitors. Bioorg. Med. Chem. Lett. 15, 4713–4716 (2005)
Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53, 240–255 (1997)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr D 60, 2126–2132 (2004)
Acknowledgements
This study was funded by the British Heart Foundation, the Wellcome Trust, the Isaac Newton Trust of the University of Cambridge and the UK Medical Research Council, with the support of the Daresbury SRS and Diamond Light Source. We acknowledge the assistance of G. Bunkóczi in data processing, H. Hong for the HPLC measurements of angiotensin, and T. Prime for analysing S-nitrosothiols.
Author information
Authors and Affiliations
Contributions
This paper is a culmination of a 20-year study initiated by R.W.C. with P.E.S.; R.W.C. guided the work throughout and wrote the paper with input from A.Z. and R.J.R.; A.Z. designed, performed and analysed all the work reported here, with Z.W., Y.Y. and, for redox studies, with M.P.M.; P.E.S. with P.L.D.S. carried out preceding studies, and F.B.P. provided samples and advice for the pre-eclamptic studies. R.J.R. advised on all aspects of this study and solved the structures. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Methods and additional references, Supplementary Table 1, Supplementary Figures 1-6 with legends and a legend for Supplementary Movie 1. (PDF 1259 kb)
Supplementary Movie 1
The movie file shows the conformational changes that occur when human angiotensinogen binds to human rennin – see Supplementary Information file for full legend. (MOV 7077 kb)
Rights and permissions
About this article
Cite this article
Zhou, A., Carrell, R., Murphy, M. et al. A redox switch in angiotensinogen modulates angiotensin release. Nature 468, 108–111 (2010). https://doi.org/10.1038/nature09505
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09505
This article is cited by
-
Decreased angiotensin receptor 1 expression in ± AT1 Knockout mice testis results in male infertility and GnRH reduction
Reproductive Biology and Endocrinology (2021)
-
A pilot study of alterations in oxidized angiotensinogen and antioxidants in pre-eclamptic pregnancy
Scientific Reports (2020)
-
Measurement of the total angiotensinogen and its reduced and oxidised forms in human plasma using targeted LC-MS/MS
Analytical and Bioanalytical Chemistry (2019)
-
Allosteric disulphide bonds as reversible mechano-sensitive switches that control protein functions in the vasculature
Biophysical Reviews (2019)
-
Logic gate aggregation of poly(N-isopropylacrylamide) nanogels with catechol substituents that respond to body heat
Polymer Journal (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.