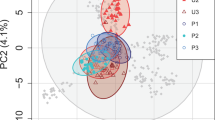
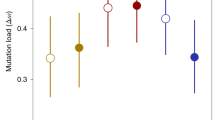
Abstract
The evolution and maintenance of sexual reproduction has puzzled biologists for decades1,2. Although this field is rich in hypotheses3,4,5, experimental evidence is scarce. Some important experiments have demonstrated differences in evolutionary rates between sexual and asexual populations6,7,8; other experiments have documented evolutionary changes in phenomena related to genetic mixing, such as recombination9,10 and selfing11. However, direct experiments of the evolution of sex within populations are extremely rare (but see ref. 12). Here we use the rotifer, Brachionus calyciflorus, which is capable of both sexual and asexual reproduction, to test recent theory13,14,15 predicting that there is more opportunity for sex to evolve in spatially heterogeneous environments. Replicated experimental populations of rotifers were maintained in homogeneous environments, composed of either high- or low-quality food habitats, or in heterogeneous environments that consisted of a mix of the two habitats. For populations maintained in either type of homogeneous environment, the rate of sex evolves rapidly towards zero. In contrast, higher rates of sex evolve in populations experiencing spatially heterogeneous environments. The data indicate that the higher level of sex observed under heterogeneity is not due to sex being less costly or selection against sex being less efficient; rather sex is sufficiently advantageous in heterogeneous environments to overwhelm its inherent costs2. Counter to some alternative theories16,17 for the evolution of sex, there is no evidence that genetic drift plays any part in the evolution of sex in these populations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bell, G. The Masterpiece of Nature: The Evolution and Genetics of Sexuality (University of California Press, 1982)
Maynard Smith, J. The Evolution of Sex (Cambridge University Press, 1978)
Otto, S. P. The evolutionary enigma of sex. Am. Nat. 174, S1–S14 (2009)
Agrawal, A. F. Evolution of sex: why do organisms shuffle their genotypes? Curr. Biol. 16, R696–R704 (2006)
Kondrashov, A. S. Classification of hypotheses on the advantage of amphimixis. J. Hered. 84, 372–387 (1993)
Colegrave, N. Sex releases the speed limit on evolution. Nature 420, 664–666 (2002)
Goddard, M. R., Godfray, H. C. J. & Burt, A. Sex increases the efficacy of natural selection in experimental yeast populations. Nature 434, 636–640 (2005)
Poon, A. & Chao, L. Drift increases the advantage of sex in RNA bacteriophage Phi 6. Genetics 166, 19–24 (2004)
Korol, A. B. & Iliadi, K. G. Increased recombination frequencies resulting from directional selection for geotaxis in Drosophila. Heredity 72, 64–68 (1994)
Otto, S. P. & Barton, N. H. Selection for recombination in small populations. Evolution 55, 1921–1931 (2001)
Morran, L. T., Parmenter, M. D. & Phillips, P. C. Mutation load and rapid adaptation favour outcrossing over self-fertilization. Nature 462, 350–352 (2009)
Wolf, H. G., Wohrmann, K. & Tomiuk, J. Experimental evidence for the adaptative value of sexual reproduction. Genetica 72, 151–159 (1987)
Lenormand, T. & Otto, S. P. The evolution of recombination in a heterogeneous environment. Genetics 156, 423–438 (2000)
Agrawal, A. F. Spatial heterogeneity and the evolution of sex in diploids. Am. Nat. 174, S54–S70 (2009)
Pylkov, K. V., Zhivotovsky, L. A. & Feldman, M. W. Migration versus mutation in the evolution of recombination under multilocus selection. Genet. Res. 71, 247–256 (1998)
Felsenstein, J. & Yokoyama, S. Evolutionary advantage of recombination. 2. Individual selection for recombination. Genetics 83, 845–859 (1976)
Keightley, P. D. & Otto, S. P. Interference among deleterious mutations favours sex and recombination in finite populations. Nature 443, 89–92 (2006)
Roze, D. & Michod, R. D. Deleterious mutations and selection for sex in finite diploid populations. Genetics 184, 1095–1112 (2010)
Kondrashov, A. S. Deleterious mutations as an evolutionary factor. 1. The advantage of recombination. Genet. Res. 44, 199–217 (1984)
Agrawal, A. F. Differences between selection on sex versus recombination in Red Queen models with diploid hosts. Evolution 63, 2131–2141 (2009)
Otto, S. P. & Nuismer, S. L. Species interactions and the evolution of sex. Science 304, 1018–1020 (2004)
Peters, A. D. & Lively, C. M. Short- and long-term benefits and detriments to recombination under antagonistic coevolution. J. Evol. Biol. 20, 1206–1217 (2007)
Hedrick, P. W. Genetic-polymorphism in heterogeneous environment—a decade later. Annu. Rev. Ecol. Syst. 17, 535–566 (1986)
Felsenstein, J. Theoretical population-genetics of variable selection and migration. Annu. Rev. Genet. 10, 253–280 (1976)
Feldman, M. W., Otto, S. P. & Christiansen, F. B. Population genetic perspectives on the evolution of recombination. Annu. Rev. Genet. 30, 261–295 (1997)
Gilbert, J. J. Specificity of crowding response that induces sexuality in the rotifer Brachionus. Limnol. Oceanogr. 48, 1297–1303 (2003)
Hill, W. G. & Robertson, A. Effects of linkage on limits to artificial selection. Genet. Res. 8, 269–294 (1966)
Williams, G. C. Sex and Evolution (Princeton University Press, 1975)
Hadany, L. & Otto, S. P. The evolution of condition-dependent sex in the face of high costs. Genetics 176, 1713–1727 (2007)
Fussmann, G. F., Ellner, S. P., Shertzer, K. W. & Hairston, N. G., Jr Crossing the Hopf bifurcation in a live predator-prey system. Science 290, 1358–1360 (2000)
Team, R. D. C. R: a Language and Environment for Statistical Computing (R Foundation for Statistical Computing Vienna, 2009)
Bates, D. & Maechler, M. lme4: Linear Mixed-Effects Models Using S4 Classes. R package version 0. 999375-31 〈http://CRAN.R-project.org/package=lme4〉 (2009)
Acknowledgements
We thank C. Kearns and N. G. Hairston Jr for providing the sediment with resting eggs from which the Brachionus cultures were established. This research was funded by the Volkswagen Foundation to L.B. (I/83 517) and the Natural Sciences and Engineering Research Council (Canada) to A.F.A.
Author information
Authors and Affiliations
Contributions
L.B. and A.F.A. conceived and designed the study, L.B. performed experiments, L.B. and A.F.A. discussed and analysed the results, and shared the writing of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-6 with legends and additional references. (PDF 5871 kb)
Rights and permissions
About this article
Cite this article
Becks, L., Agrawal, A. Higher rates of sex evolve in spatially heterogeneous environments. Nature 468, 89–92 (2010). https://doi.org/10.1038/nature09449
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09449
This article is cited by
-
Adaptation of a prey population to increasing predation risks
Hydrobiologia (2023)
-
Genetic variation in released gametes produces genetic diversity in the offspring of the broadcast spawning coral Acropora tenuis
Scientific Reports (2022)
-
Evolution of a primary consumer in response to low and high food availability shapes life history traits and population demography
Hydrobiologia (2022)
-
Recombination mapping of the Brazilian stingless bee Frieseomelitta varia confirms high recombination rates in social hymenoptera
BMC Genomics (2021)
-
Enlarge or die! An auxospore perspective on diatom diversification
Organisms Diversity & Evolution (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.