Abstract
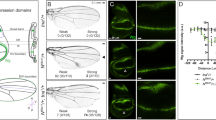
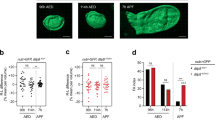
Genes include cis-regulatory regions that contain transcriptional enhancers. Recent reports have shown that developmental genes often possess multiple discrete enhancer modules that drive transcription in similar spatio-temporal patterns1,2,3,4: primary enhancers located near the basal promoter and secondary, or ‘shadow’, enhancers located at more remote positions. It has been proposed that the seemingly redundant activity of primary and secondary enhancers contributes to phenotypic robustness1,5. We tested this hypothesis by generating a deficiency that removes two newly discovered enhancers of shavenbaby (svb, a transcript of the ovo locus), a gene encoding a transcription factor that directs development of Drosophila larval trichomes6. At optimal temperatures for embryonic development, this deficiency causes minor defects in trichome patterning. In embryos that develop at both low and high extreme temperatures, however, absence of these secondary enhancers leads to extensive loss of trichomes. These temperature-dependent defects can be rescued by a transgene carrying a secondary enhancer driving transcription of the svb cDNA. Finally, removal of one copy of wingless, a gene required for normal trichome patterning7, causes a similar loss of trichomes only in flies lacking the secondary enhancers. These results support the hypothesis that secondary enhancers contribute to phenotypic robustness in the face of environmental and genetic variability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hong, J. W., Hendrix, D. A. & Levine, M. S. Shadow enhancers as a source of evolutionary novelty. Science 321, 1314 (2008)
Werner, T., Hammer, A., Wahlbuhl, M., Bosl, M. R. & Wegner, M. Multiple conserved regulatory elements with overlapping functions determine Sox10 expression in mouse embryogenesis. Nucleic Acids Res. 35, 6526–6538 (2007)
Zeitlinger, J. et al. Whole-genome ChIP-chip analysis of Dorsal, Twist, and Snail suggests integration of diverse patterning processes in the Drosophila embryo. Genes Dev. 21, 385–390 (2007)
Jeong, Y., El-Jaick, K., Roessler, E., Muenke, M. & Epstein, D. J. A functional screen for sonic hedgehog regulatory elements across a 1 Mb interval identifies long-range ventral forebrain enhancers. Development 133, 761–772 (2006)
Perry, M. W., Cande, J. D., Boettiger, A. N. & Levine, M. Evolution of insect dorsoventral patterning mechanisms. Cold Spring Harb. Symp. Quant. Biol. advance online publication, 10.1101/sqb.2009.74.021 (20 October 2009)
Payre, F., Vincent, A. & Carreno, S. ovo/svb integrates Wingless and DER pathways to control epidermis differentiation. Nature 400, 271–275 (1999)
Bokor, P. & DiNardo, S. The roles of hedgehog, wingless and lines in patterning the dorsal epidermis in Drosophila . Development 122, 1083–1092 (1996)
Overton, P. M., Chia, W. & Buescher, M. The Drosophila HMG-domain proteins SoxNeuro and Dichaete direct trichome formation via the activation of shavenbaby and the restriction of Wingless pathway activity. Development 134, 2807–2813 (2007)
Chanut-Delalande, H., Fernandes, I., Roch, F., Payre, F. & Plaza, S. Shavenbaby couples patterning to epidermal cell shape control. PLoS Biol. 4, e290 (2006)
Fernandes, I. et al. Zona pellucida domain proteins remodel the apical compartment for localized cell shape changes. Dev. Cell 18, 64–76 (2010)
McGregor, A. P. et al. Morphological evolution through multiple cis-regulatory mutations at a single gene. Nature 448, 587–590 (2007)
Sucena, E., Delon, I., Jones, I., Payre, F. & Stern, D. L. Regulatory evolution of shavenbaby/ovo underlies multiple cases of morphological parallelism. Nature 424, 935–938 (2003)
Crane-Robinson, C., Dragan, A. I. & Read, C. M. Defining the thermodynamics of protein/DNA complexes and their components using micro-calorimetry. Methods Mol. Biol. 543, 625–651 (2009)
Hochachka, P. W. & Somero, G. N. Biochemical Adaptation: Mechanism and Process in Physiological Evolution (Oxford Univ. Press, 2002)
Powsner, L. The effects of temperature on the durations of the developmental stages of Drosophila melanogaster . Physiol. Zool. 8, 474–520 (1935)
Delon, I., Chanut-Delalande, H. & Payre, F. The Ovo/Shavenbaby transcription factor specifies actin remodelling during epidermal differentiation in Drosophila . Mech. Dev. 120, 747–758 (2003)
Nijhout, H. F. & Davidowitz, G. in Developmental Instability: Causes and Consequences (ed. M. Polak) Ch. 1 (Oxford Univ. Press, 2003)
Boettiger, A. N. & Levine, M. Synchronous and stochastic patterns of gene activation in the Drosophila embryo. Science 325, 471–473 (2009)
Cretekos, C. J. et al. Regulatory divergence modifies limb length between mammals. Genes Dev. 22, 141–151 (2008)
Xiong, N., Kang, C. & Raulet, D. H. Redundant and unique roles of two enhancer elements in the TCRγ locus in gene regulation and γδ T cell development. Immunity 16, 453–463 (2002)
Li, X., Cassidy, J. J., Reinke, C. A., Fischboeck, S. & Carthew, R. W. A microRNA imparts robustness against environmental fluctuation during development. Cell 137, 273–282 (2009)
Crickmore, M. A., Ranade, V. & Mann, R. S. Regulation of Ubx expression by epigenetic enhancer silencing in response to Ubx levels and genetic variation. PLoS Genet. 5, e1000633 (2009)
Raser, J. M. & O’Shea, E. K. Noise in gene expression: origins, consequences, and control. Science 309, 2010–2013 (2005)
van den Heuvel, M., Harryman-Samos, C., Klingensmith, J., Perrimon, N. & Nusse, R. Mutations in the segment polarity genes wingless and porcupine impair secretion of the wingless protein. EMBO J. 12, 5293–5302 (1993)
Bischof, J., Maeda, R. K., Hediger, M., Karch, F. & Basler, K. An optimized transgenesis system for Drosophila using germ-line-specific φC31 integrases. Proc. Natl Acad. Sci. USA 104, 3312–3317 (2007)
Parks, A. L. et al. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nature Genet. 36, 288–292 (2004)
Acknowledgements
We thank D. Chien and D. Erezyilmaz for assistance with early experiments, L. Kruglyak, S. Levin and S. Tavazoie for helpful comments on the manuscript, and E. Wieschaus for providing the wg mutant flies. This work was supported by The Pew Charitable Trusts Latin American Fellows Program in the Biomedical Sciences Fellowship to N.F., Agence Nationale de la Recherche (Blanc 2008, Netoshape) to F.P., and NIH (GM063622-06A1) and NSF (IOS-0640339) grants to D.L.S.
Author information
Authors and Affiliations
Contributions
N.F., G.K.D. and D.L.S. designed the experiments. N.F., G.K.D., D.V., S.W., F.P. and D.L.S. performed the experimental work. N.F. and D.L.S. wrote the manuscript. G.K.D. and F.P. commented on the manuscript at all stages.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-3 with legends. (PDF 2343 kb)
Rights and permissions
About this article
Cite this article
Frankel, N., Davis, G., Vargas, D. et al. Phenotypic robustness conferred by apparently redundant transcriptional enhancers. Nature 466, 490–493 (2010). https://doi.org/10.1038/nature09158
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09158
This article is cited by
-
Deep learning connects DNA traces to transcription to reveal predictive features beyond enhancer–promoter contact
Nature Communications (2021)
-
Dissection of the Fgf8 regulatory landscape by in vivo CRISPR-editing reveals extensive intra- and inter-enhancer redundancy
Nature Communications (2021)
-
Evolution, structure and emerging roles of C1ORF112 in DNA replication, DNA damage responses, and cancer
Cellular and Molecular Life Sciences (2021)
-
Inter-embryo gene expression variability recapitulates the hourglass pattern of evo-devo
BMC Biology (2020)
-
SINE Retrotransposon variation drives Ecotypic disparity in natural populations of Coilia nasus
Mobile DNA (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.