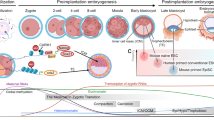
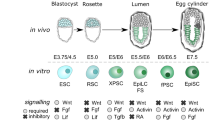
Abstract
During early mammalian development, as the pluripotent cells that give rise to all of the tissues of the body proliferate and expand in number, they pass through transition states marked by a stepwise restriction in developmental potential and by changes in the expression of key regulatory genes. Recent findings show that cultured stem-cell lines derived from different stages of mouse development can mimic these transition states. They further reveal that there is a high degree of heterogeneity and plasticity in pluripotent populations in vitro and that these properties are modulated by extrinsic signalling. Understanding the extrinsic control of plasticity will guide efforts to use human pluripotent stem cells in research and therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Thomson, J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998).
Reubinoff, B. E., Pera, M. F., Fong, C. Y., Trounson, A. & Bongso, A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro . Nature Biotechnol. 18, 399–404 (2000).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Bibikova, M., Laurent, L. C., Ren, B., Loring, J. F. & Fan, J. B. Unraveling epigenetic regulation in embryonic stem cells. Cell Stem Cell 2, 123–134 (2008).
Chambers, I. & Tomlinson, S. R. The transcriptional foundation of pluripotency. Development 136, 2311–2322 (2009).
Nishikawa, S., Jakt, L. M. & Era, T. Embryonic stem-cell culture as a tool for developmental cell biology. Nature Rev. Mol. Cell Biol. 8, 502–507 (2007).
Tam, P. P. & Loebel, D. A. Gene function in mouse embryogenesis: get set for gastrulation. Nature Rev. Genet. 8, 368–381 (2007).
Assou, S. et al. A meta-analysis of human embryonic stem cells transcriptome integrated into a web-based expression atlas. Stem Cells 25, 961–973 (2007).
Adewumi, O. et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nature Biotechnol. 25, 803–816 (2007).
Henderson, J. K. et al. Preimplantation human embryos and embryonic stem cells show comparable expression of stage-specific embryonic antigens. Stem Cells 20, 329–337 (2002).
Cauffman, G., Van de Velde, H., Liebaers, I. & Van Steirteghem, A. Oct-4 mRNA and protein expression during human preimplantation development. Mol. Hum. Reprod. 11, 173–181 (2005).
Huntriss, J. et al. Expression of mRNAs for DNA methyltransferases and methyl-CpG-binding proteins in the human female germ line, preimplantation embryos, and embryonic stem cells. Mol. Reprod. Dev. 67, 323–336 (2004).
Adjaye, J. et al. Primary differentiation in the human blastocyst: comparative molecular portraits of inner cell mass and trophectoderm cells. Stem Cells 23, 1514–1525 (2005).
Rathjen, J. et al. Formation of a primitive ectoderm like cell population, EPL cells, from ES cells in response to biologically derived factors. J. Cell Sci. 112, 601–612 (1999).
Brons, I. G. et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448, 191–195 (2007).
Tesar, P. J. et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448, 196–199 (2007). References 17 and 18 describe the derivation of pluripotent stem-cell lines with novel properties from the post-implantation epiblast in mice.
Nichols, J., Silva, J., Roode, M. & Smith, A. Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development 136, 3215–3222 (2009).
Bao, S. et al. Epigenetic reversion of post-implantation epiblast to pluripotent embryonic stem cells. Nature 461, 1292–1295 (2009). This study shows the conversion of EpiSCs to ES cells.
Chou, Y. F. et al. The growth factor environment defines distinct pluripotent ground states in novel blastocyst-derived stem cells. Cell 135, 449–461 (2008).
Nichols, J. & Smith, A. Naive and primed pluripotent states. Cell Stem Cell 4, 487–492 (2009).
Vallier, L. et al. Activin/Nodal signalling maintains pluripotency by controlling Nanog expression. Development 136, 1339–1349 (2009).
Clark, A. T. et al. Human STELLAR, NANOG, and GDF3 genes are expressed in pluripotent cells and map to chromosome 12p13, a hotspot for teratocarcinoma. Stem Cells 22, 169–179 (2004).
Chan, K. K. et al. KLF4 and PBX1 directly regulate NANOG expression in human embryonic stem cells. Stem Cells 27, 2114–2125 (2009).
Hayashi, K., Lopes, S. M., Tang, F. & Surani, M. A. Dynamic equilibrium and heterogeneity of mouse pluripotent stem cells with distinct functional and epigenetic states. Cell Stem Cell 3, 391–401 (2008). This paper provides evidence for the interconversion of stem-cell states in mouse ES-cell cultures.
Guo, G. et al. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 136, 1063–1069 (2009).
Babaie, Y. et al. Analysis of Oct4-dependent transcriptional networks regulating self-renewal and pluripotency in human embryonic stem cells. Stem Cells 25, 500–510 (2007).
Greber, B. et al. Conserved and divergent roles of FGF signaling in mouse epiblast stem cells and human embryonic stem cells. Cell Stem Cell 6, 215–226 (2010).
Ogawa, K. et al. Activin–Nodal signaling is involved in propagation of mouse embryonic stem cells. J. Cell Sci. 120, 55–65 (2007).
James, D., Levine, A. J., Besser, D. & Hemmati-Brivanlou, A. TGFβ/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development 132, 1273–1282 (2005).
Pera, M. F. & Trounson, A. O. Human embryonic stem cells: prospects for development. Development 131, 5515–5525 (2004).
Eakin, G. S. & Behringer, R. R. Diversity of germ layer and axis formation among mammals. Semin. Cell Dev. Biol. 15, 619–629 (2004).
Murry, C. E. & Keller, G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132, 661–680 (2008).
Pera, M. F., Cooper, S., Mills, J. & Parrington, J. M. Isolation and characterization of a multipotent clone of human embryonal carcinoma cells. Differentiation 42, 10–23 (1989).
Pera, M. F., Reubinoff, B. & Trounson, A. Human embryonic stem cells. J. Cell Sci. 113, 5–10 (2000).
Vallier, L., Alexander, M. & Pedersen, R. A. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J. Cell Sci. 118, 4495–4509 (2005).
Vallier, L., Reynolds, D. & Pedersen, R. A. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev. Biol. 275, 403–421 (2004).
Xiao, L., Yuan, X. & Sharkis, S. J. Activin A maintains self-renewal and regulates fibroblast growth factor, Wnt, and bone morphogenic protein pathways in human embryonic stem cells. Stem Cells 24, 1476–1486 (2006).
Greber, B., Lehrach, H. & Adjaye, J. Fibroblast growth factor 2 modulates transforming growth factor β signaling in mouse embryonic fibroblasts and human ESCs (hESCs) to support hESC self-renewal. Stem Cells 25, 455–464 (2007).
Xu, R. H. et al. NANOG is a direct target of TGFβ/activin-mediated SMAD signaling in human ESCs. Cell Stem Cell 3, 196–206 (2008). This study reveals how TGF- β -mediated signalling affects the transcriptional regulation of pluripotency.
Dvash, T., Sharon, N., Yanuka, O. & Benvenisty, N. Molecular analysis of LEFTY-expressing cells in early human embryoid bodies. Stem Cells 25, 465–472 (2007).
Takaoka, K. et al. The mouse embryo autonomously acquires anterior–posterior polarity at implantation. Dev. Cell 10, 451–459 (2006).
Chazaud, C., Yamanaka, Y., Pawson, T. & Rossant, J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2–MAPK pathway. Dev. Cell 10, 615–624 (2006).
Shen, M. M. Nodal signaling: developmental roles and regulation. Development 134, 1023–1034 (2007).
Levine, A. J. & Brivanlou, A. H. GDF3 at the crossroads of TGF-β signaling. Cell Cycle 5, 1069–1073 (2006).
Chen, C. et al. The Vg1-related protein Gdf3 acts in a Nodal signaling pathway in the pre-gastrulation mouse embryo. Development 133, 319–329 (2006).
Levine, A. J. & Brivanlou, A. H. GDF3, a BMP inhibitor, regulates cell fate in stem cells and early embryos. Development 133, 209–216 (2006). References 47 and 48 elucidate the functions of GDF3, a putative autocrine maintenance factor for ES cells.
Levine, A. J., Levine, Z. J. & Brivanlou, A. H. GDF3 is a BMP inhibitor that can activate Nodal signaling only at very high doses. Dev. Biol. 325, 43–48 (2009).
Hannan, N. R., Jamshidi, P., Pera, M. F. & Wolvetang, E. J. BMP-11 and myostatin support undifferentiated growth of human embryonic stem cells in feeder-free cultures. Cloning Stem Cells 11, 427–435 (2009).
Itsykson, P. et al. Derivation of neural precursors from human embryonic stem cells in the presence of noggin. Mol. Cell. Neurosci. 30, 24–36 (2005).
Pera, M. F. et al. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J. Cell Sci. 117, 1269–1280 (2004).
Xu, R. H. et al. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nature Methods 2, 185–190 (2005).
Ying, Q. L., Nichols, J., Chambers, I. & Smith, A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 115, 281–292 (2003).
Sirard, C. et al. Targeted disruption in murine cells reveals variable requirement for Smad4 in transforming growth factor β-related signaling. J. Biol. Chem. 275, 2063–2070 (2000).
Mishina, Y., Suzuki, A., Ueno, N. & Behringer, R. R. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 9, 3027–3037 (1995).
Winnier, G., Blessing, M., Labosky, P. A. & Hogan, B. L. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 9, 2105–2116 (1995).
Peerani, R. et al. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 26, 4744–4755 (2007). This paper uncovers autocrine and paracrine factors involved in human ES-cell regulation.
Amit, M. et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev. Biol. 227, 271–278 (2000).
Chase, L. G. & Firpo, M. T. Development of serum-free culture systems for human embryonic stem cells. Curr. Opin. Chem. Biol. 11, 367–372 (2007).
Dvorak, P. et al. Expression and potential role of fibroblast growth factor 2 and its receptors in human embryonic stem cells. Stem Cells 23, 1200–1211 (2005).
Li, J. et al. MEK/ERK signaling contributes to the maintenance of human embryonic stem cell self-renewal. Differentiation 75, 299–307 (2007).
Kang, H. B. et al. Basic fibroblast growth factor activates ERK and induces c-fos in human embryonic stem cell line MizhES1. Stem Cells Dev. 14, 395–401 (2005).
Burdon, T., Stracey, C., Chambers, I., Nichols, J. & Smith, A. Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells. Dev. Biol. 210, 30–43 (1999).
Wilder, P. J. et al. Inactivation of the FGF-4 gene in embryonic stem cells alters the growth and/or the survival of their early differentiated progeny. Dev. Biol. 192, 614–629 (1997).
Tanaka, S., Kunath, T., Hadjantonakis, A. K., Nagy, A. & Rossant, J. Promotion of trophoblast stem cell proliferation by FGF4. Science 282, 2072–2075 (1998).
Hamazaki, T., Kehoe, S. M., Nakano, T. & Terada, N. The Grb2/Mek pathway represses Nanog in murine embryonic stem cells. Mol. Cell. Biol. 26, 7539–7549 (2006).
Kunath, T. et al. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development 134, 2895–2902 (2007).
Bendall, S. C. et al. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro . Nature 448, 1015–1021 (2007).
Stojkovic, P. et al. An autogeneic feeder cell system that efficiently supports growth of undifferentiated human embryonic stem cells. Stem Cells 23, 306–314 (2005).
McLean, A. B. et al. Activin A efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells 25, 29–38 (2007).
Pebay, A. et al. Essential roles of sphingosine-1-phosphate and platelet-derived growth factor in the maintenance of human embryonic stem cells. Stem Cells 23, 1541–1548 (2005).
Brill, L. M. et al. Phosphoproteomic analysis of human embryonic stem cells. Cell Stem Cell 5, 204–213 (2009). This study takes a proteomics approach to investigating signal transduction in human ES cells.
Nishikawa, S. I., Nishikawa, S., Hirashima, M., Matsuyoshi, N. & Kodama, H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development 125, 1747–1757 (1998).
Wang, L. et al. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood 110, 4111–4119 (2007).
Wang, X., Lin, G., Martins-Taylor, K., Zeng, H. & Xu, R. H. Inhibition of caspase-mediated anoikis is critical for basic fibroblast growth factor-sustained culture of human pluripotent stem cells. J. Biol. Chem. 284, 34054–34064 (2009).
Wong, R. C., Tellis, I., Jamshidi, P., Pera, M. & Pebay, A. Anti-apoptotic effect of sphingosine-1-phosphate and platelet-derived growth factor in human embryonic stem cells. Stem Cells Dev. 16, 989–1001 (2007).
Sato, N., Meijer, L., Skaltsounis, L., Greengard, P. & Brivanlou, A. H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature Med. 10, 55–63 (2004).
Dravid, G. et al. Defining the role of Wnt/β-catenin signaling in the survival, proliferation, and self-renewal of human embryonic stem cells. Stem Cells 23, 1489–1501 (2005).
Lu, J., Hou, R., Booth, C. J., Yang, S. H. & Snyder, M. Defined culture conditions of human embryonic stem cells. Proc. Natl Acad. Sci. USA 103, 5688–5693 (2006).
Miyabayashi, T. et al. Wnt/β-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proc. Natl Acad. Sci. USA 104, 5668–5673 (2007).
Liu, P. et al. Requirement for Wnt3 in vertebrate axis formation. Nature Genet. 22, 361–365 (1999).
Kemp, C., Willems, E., Abdo, S., Lambiv, L. & Leyns, L. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev. Dyn. 233, 1064–1075 (2005).
Niwa, H., Ogawa, K., Shimosato, D. & Adachi, K. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature 460, 118–122 (2009).
Silva, J. & Smith, A. Capturing pluripotency. Cell 132, 532–536 (2008).
Ying, Q. L. et al. The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 (2008). This study shows that mouse ES cells default to the state of self-renewal when the signalling pathways that activate their differentiation are suppressed.
Enver, T. et al. Cellular differentiation hierarchies in normal and culture-adapted human embryonic stem cells. Hum. Mol. Genet. 14, 3129–3140 (2005).
Hough, S. R., Laslett, A. L., Grimmond, S. B., Kolle, G. & Pera, M. F. A continuum of cell states spans pluripotency and lineage commitment in human embryonic stem cells. PLoS ONE 4, e7708 (2009).
Laslett, A. L. et al. Transcriptional analysis of early lineage commitment in human embryonic stem cells. BMC Dev. Biol. 7, 12 (2007).
Kolle, G. et al. Identification of human embryonic stem cell surface markers by combined membrane-polysome translation state array analysis and immunotranscriptional profiling. Stem Cells 27, 2446–2456 (2009).
Hanna, J. et al. Metastable pluripotent states in NOD-mouse-derived ESCs. Cell Stem Cell 4, 513–524 (2009).
Lander, A. D. The 'stem cell' concept: is it holding us back? J. Biol. 8, 70 (2009). An overview of emerging concepts of stemness in cell lineages.
Rossant, J. & Tam, P. P. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development 136, 701–713 (2009).
Evans, M. J. & Kaufman, M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156 (1981).
Martin, G. R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl Acad. Sci. USA 78, 7634–7638 (1981).
Nichols, J., Evans, E. P. & Smith, A. G. Establishment of germ-line-competent embryonic stem (ES) cells using differentiation inhibiting activity. Development 110, 1341–1348 (1990).
Kunath, T. et al. Imprinted X-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts. Development 132, 1649–1661 (2005).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reprints and permissions information is available at http://www.nature.com/reprints.
Rights and permissions
About this article
Cite this article
Pera, M., Tam, P. Extrinsic regulation of pluripotent stem cells. Nature 465, 713–720 (2010). https://doi.org/10.1038/nature09228
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09228
This article is cited by
-
Glutamate secretion by embryonic stem cells as an autocrine signal to promote proliferation
Scientific Reports (2023)
-
Maternal Undernutrition Induces Cell Signalling and Metabolic Dysfunction in Undifferentiated Mouse Embryonic Stem Cells
Stem Cell Reviews and Reports (2023)
-
NETISCE: a network-based tool for cell fate reprogramming
npj Systems Biology and Applications (2022)
-
A dose-dependent response to MEK inhibition determines hypoblast fate in bovine embryos
BMC Developmental Biology (2019)
-
Role of the Extracellular Matrix in Stem Cell Maintenance
Current Stem Cell Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.