Abstract
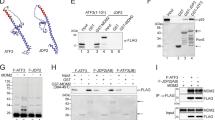
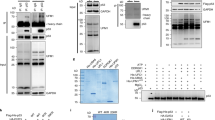
The tumour suppressor ARF is specifically required for p53 activation under oncogenic stress1,2,3,4,5,6. Recent studies showed that p53 activation mediated by ARF, but not that induced by DNA damage, acts as a major protection against tumorigenesis in vivo under certain biological settings7,8, suggesting that the ARF–p53 axis has more fundamental functions in tumour suppression than originally thought. Because ARF is a very stable protein in most human cell lines, it has been widely assumed that ARF induction is mediated mainly at the transcriptional level and that activation of the ARF–p53 pathway by oncogenes is a much slower and largely irreversible process by comparison with p53 activation after DNA damage. Here we report that ARF is very unstable in normal human cells but that its degradation is inhibited in cancerous cells. Through biochemical purification, we identified a specific ubiquitin ligase for ARF and named it ULF. ULF interacts with ARF both in vitro and in vivo and promotes the lysine-independent ubiquitylation and degradation of ARF. ULF knockdown stabilizes ARF in normal human cells, triggering ARF-dependent, p53-mediated growth arrest. Moreover, nucleophosmin (NPM) and c-Myc, both of which are commonly overexpressed in cancer cells, are capable of abrogating ULF-mediated ARF ubiquitylation through distinct mechanisms, and thereby promote ARF stabilization in cancer cells. These findings reveal the dynamic feature of the ARF–p53 pathway and suggest that transcription-independent mechanisms are critically involved in ARF regulation during responses to oncogenic stress.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Data deposits
The full-length ULF sequence is deposited in GenBank under accession number EU489742.
References
Lowe, S. W. & Sherr, C. J. Tumor suppression by Ink4a-Arf: progress and puzzles. Curr. Opin. Genet. Dev. 13, 77–83 (2003)
Gil, J. & Peters, G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: all for one or one for all. Nature Rev. Mol. Cell Biol. 7, 667–677 (2006)
Matheu, A., Maraver, A. & Serrano, M. The Arf/p53 pathway in cancer and aging. Cancer Res. 68, 6031–6034 (2008)
Brooks, C. L. & Gu, W. p53 ubiquitination: Mdm2 and beyond. Mol. Cell 21, 307–315 (2006)
Sherr, C. J. Divorcing ARF and p53: an unsettled case. Nature Rev. Cancer 6, 663–673 (2006)
Kruse, J.-P. & Gu, W. Modes of p53 regulation. Cell 137, 609–622 (2009)
Christophorou, M. A., Ringshausen, I., Finch, A. J., Swigart, L. B. & Evan, G. I. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature 443, 214–217 (2006)
Martins, C. P., Brown-Swigart, L. & Evan, G. I. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell 127, 1323–1334 (2006)
Kuo, M. L., den Besten, W., Bertwistle, D., Roussel, M. F. & Sherr, C. J. N-terminal polyubiquitination and degradation of the Arf tumor suppressor. Genes Dev. 18, 1862–1874 (2004)
Itahana, K. et al. Tumor suppressor ARF degrades B23, a nucleolar protein involved in ribosome biogenesis and cell proliferation. Mol. Cell 12, 1151–1164 (2003)
Korgaonkar, C. et al. Nucleophosmin (B23) targets ARF to nucleoli and inhibits its function. Mol. Cell. Biol. 25, 1258–1271 (2005)
Brady, S. N., Yu, Y., Maggi, L. B. & Weber, J. D. ARF impedes NPM/B23 shuttling in an Mdm2-sensitive tumor suppressor pathway. Mol. Cell. Biol. 24, 9327–9338 (2004)
Bertwistle, D., Sugimoto, M. & Sherr, C. J. Physical and functional interactions of the Arf tumor suppressor protein with nucleophosmin/B23. Mol. Cell. Biol. 24, 985–996 (2004)
Chen, D. L. et al. ARF-BP1/mule is a critical mediator of the ARF tumor suppressor. Cell 121, 1071–1083 (2005)
Moulin, S., Llanos, S., Kim, S. H. & Peters, G. Binding to nucleophosmin determines the localization of human and chicken ARF but not its impact on p53. Oncogene 27, 2382–2389 (2008)
Colombo, E. et al. Nucleophosmin is required for DNA integrity and p19Arf protein stability. Mol. Cell. Biol. 25, 8874–8886 (2005)
Grisendi, S. et al. Role of nucleophosmin in embryonic development and tumorigenesis. Nature 437, 147–153 (2005)
Lee, J. W., Choi, H. S., Gyuris, J., Brent, R. & Moore, D. D. Two classes of proteins dependent on either the presence or absence of thyroid hormone for interaction with the thyroid hormone receptor. Mol. Endocrinol. 9, 243–254 (1995)
den Besten, W., Kuo, M. L., Williams, R. T. & Sherr, C. J. Myeloid leukemia-associated nucleophosmin mutants perturb p53-dependent and independent activities of the Arf tumor suppressor protein. Cell Cycle 4, 1593–1598 (2005)
Colombo, E. et al. Delocalization and destabilization of the Arf tumor suppressor by the leukemia-associated NPM mutant. Cancer Res. 66, 3044–3050 (2006)
Cheng, K. et al. The leukemia-associated cytoplasmic nucleophosmin mutant is an oncogene with paradoxical functions: Arf inactivation and induction of cellular senescence. Oncogene 26, 7391–7400 (2007)
Feuerstein, N. & Mond, J. J. ‘Numatrin,’ a nuclear matrix protein associated with induction of proliferation in B lymphocytes. J. Biol. Chem. 262, 11389–11397 (1987)
Nozawa, Y., vanBelzen, N., vanderMade, A. C. J., Dinjens, W. N. M. & Bosman, F. T. Expression of nucleophosmin/B23 in normal and neoplastic colorectal mucosa. J. Pathol. 178, 48–52 (1996)
Falini, B. et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N. Engl. J. Med. 352, 254–266 (2005)
Quentmeier, H. et al. Cell line OCI/AML3 bears exon-12 NPM gene mutation-A and cytoplasmic expression of nucleophosmin. Leukemia 19, 1760–1767 (2005)
Grisendi, S., Mecucci, C., Falini, B. & Pandolfi, P. P. Nucleophosmin and cancer. Nature Rev. Cancer 6, 493–505 (2006)
Saporita, A. J., Maggi, L. B., Apicelli, A. J. & Weber, J. D. Therapeutic targets in the ARF tumor suppressor pathway. Curr. Med. Chem. 14, 1815–1827 (2007)
Finak, G. et al. Stromal gene expression predicts clinical outcome in breast cancer. Nature Med. 14, 518–527 (2008)
Buchholz, M. et al. Transcriptome analysis of microdissected pancreatic intraepithelial neoplastic lesions. Oncogene 44, 6626–6636 (2005)
Zindy, F. et al. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 12, 2424–2433 (1998)
Abide, W. M. & Gu, W. p53-Dependent and p53-independent activation of autophagy by ARF. Cancer Res. 68, 352–357 (2006)
Li, M. et al. Mono- versus polyubiquitination: differential control of p53 fate by Mdm2. Science 302, 1972–1975 (2003)
Acknowledgements
We thank R. Baer and R. Dalla-Favera for critical comments on this study; B. Falini and J. B. Yoon for providing reagents; W. Z. Zhang for helping in mass spectrometry analysis; E. McIntush for developing the anti-ULF antibody. This study was supported by grants from National Institutes of Health/National Cancer Institute, the Leukemia and Lymphoma Society and NSFC-30628028. W.G. is an Ellison Medical Foundation Senior Scholar in aging.
Author Contributions The experiments were conceived and designed by D.C. and W.G. Experiments were performed mainly by D.C. Protein identification, mass spectrometric analysis and cloning were performed by D.C., J.S., W.Z. and J.Q. The paper was written by D.C. and W.G.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-13 with legends. (PDF 796 kb)
Rights and permissions
About this article
Cite this article
Chen, D., Shan, J., Zhu, WG. et al. Transcription-independent ARF regulation in oncogenic stress-mediated p53 responses. Nature 464, 624–627 (2010). https://doi.org/10.1038/nature08820
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08820
This article is cited by
-
The expression level of ARF and p53 in AML patients, and their relation to patients' outcome
Egyptian Journal of Medical Human Genetics (2023)
-
Control of TGFβ signalling by ubiquitination independent function of E3 ubiquitin ligase TRIP12
Cell Death & Disease (2023)
-
CRL2-KLHDC3 E3 ubiquitin ligase complex suppresses ferroptosis through promoting p14ARF degradation
Cell Death & Differentiation (2022)
-
CRL2KLHDC3 mediates p14ARF N-terminal ubiquitylation degradation to promote non-small cell lung carcinoma progression
Oncogene (2022)
-
MYC: a multipurpose oncogene with prognostic and therapeutic implications in blood malignancies
Journal of Hematology & Oncology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.