Abstract
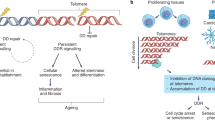
The study of human genetic disorders and mutant mouse models has provided evidence that genome maintenance mechanisms, DNA damage signalling and metabolic regulation cooperate to drive the ageing process. In particular, age-associated telomere damage, diminution of telomere 'capping' function and associated p53 activation have emerged as prime instigators of a functional decline of tissue stem cells and of mitochondrial dysfunction that adversely affect renewal and bioenergetic support in diverse tissues. Constructing a model of how telomeres, stem cells and mitochondria interact with key molecules governing genome integrity, 'stemness' and metabolism provides a framework for how diverse factors contribute to ageing and age-related disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kenyon, C. The plasticity of aging: insights from long-lived mutants. Cell 120, 449–460 (2005).
Guarente, L. Mitochondria — a nexus for aging, calorie restriction, and sirtuins? Cell 132, 171–176 (2008).
Finkel, T., Serrano, M. & Blasco, M. A. The common biology of cancer and ageing. Nature 448, 767–774 (2007).
Campisi, J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522 (2005).
Kirkwood, T. B. Understanding the odd science of aging. Cell 120, 437–447 (2005).
Arrighi, H. M., McLaughlin, T. & Leibman, C. Prevalence and impact of dementia-related functional limitations in the United States, 2001 to 2005. Alzheimer Dis. Assoc. Disord. doi:10.1097/WAD.0b013e3181a1a87d (in the press).
DePinho, R. A. The age of cancer. Nature 408, 248–254 (2000).
Sharpless, N. E. & DePinho, R. A. How stem cells age and why this makes us grow old. Nature Rev. Mol. Cell Biol. 8, 703–713 (2007).
Rossi, D. J., Jamieson, C. H. & Weissman, I. L. Stems cells and the pathways to aging and cancer. Cell 132, 681–696 (2008).
Zhao, C., Deng, W. & Gage, F. H. Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660 (2008).
Linton, P. J. & Dorshkind, K. Age-related changes in lymphocyte development and function. Nature Immunol. 5, 133–139 (2004).
Kollman, C. et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood 98, 2043–2051 (2001).
Gage, F. H. Mammalian neural stem cells. Science 287, 1433–1438 (2000).
Maslov, A. Y., Barone, T. A., Plunkett, R. J. & Pruitt, S. C. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J. Neurosci. 24, 1726–1733 (2004).
Molofsky, A. V. et al. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature 443, 448–452 (2006).
Enwere, E. et al. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J. Neurosci. 24, 8354–8365 (2004).
Cerletti, M., Shadrach, J. L., Jurga, S., Sherwood, R. & Wagers, A. J. Regulation and function of skeletal muscle stem cells. Cold Spring Harb. Symp. Quant. Biol. 73, 317–322 (2008).
Di Iorio, A. et al. Sarcopenia: age-related skeletal muscle changes from determinants to physical disability. Int. J. Immunopathol. Pharmacol. 19, 703–719 (2006).
Gopinath, S. D. & Rando, T. A. Aging of the skeletal muscle stem cell niche. Aging Cell 7, 590–598 (2008).
Conboy, I. M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 (2005).
Paik, J. H. et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell 5, 540–553 (2009).
Tothova, Z. & Gilliland, D. G. FoxO transcription factors and stem cell homeostasis: insights from the hematopoietic system. Cell Stem Cell 1, 140–152 (2007).
Gan, B. et al. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc. Natl Acad. Sci. USA 105, 19384–19389 (2008).
Yilmaz, O. H. et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 441, 475–482 (2006).
Selman, C. et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326, 140–144 (2009).
Rudolph, K. L. et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96, 701–712 (1999). This paper describes the effect of critically short telomeres on lifespan and stress response in telomerase knockout mice. Mice with short telomeres have a reduced lifespan and a diminished regenerative capacity when stressed, such as by wound healing or haematopoietic ablation.
Chin, L. et al. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97, 527–538 (1999). This paper shows that p53 mediates the cellular response to telomere dysfunction in both normal and neoplastic cells and that p53 deficiency ameliorates degenerative phenotypes.
Maier, B. et al. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 18, 306–319 (2004).
Tyner, S. D. et al. p53 mutant mice that display early ageing-associated phenotypes. Nature 415, 45–53 (2002). References 28 and 29 describe the pro-ageing phenotype in mice with hyperactive p53.
Kim, W. Y. & Sharpless, N. E. The regulation of INK4/ARF in cancer and aging. Cell 127, 265–275 (2006).
Chen, M. L. et al. Erythroid dysplasia, megaloblastic anemia, and impaired lymphopoiesis arising from mitochondrial dysfunction. Blood 114, 4045–4053 (2009).
Liu, J. et al. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature 459, 387–392 (2009). This paper describes how in mice deficient in BMI1, mitochondrial dysfunction increases ROS levels and activates the DNA damage response, which can be partially rescued by antioxidant treatment.
Tothova, Z. et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128, 325–339 (2007).
Passos, J. F., Saretzki, G. & von Zglinicki, T. DNA damage in telomeres and mitochondria during cellular senescence: is there a connection? Nucleic Acids Res. 35, 7505–7513 (2007).
McClintock, B. The behavior in successive nuclear divisions of a chromosome broken at meiosis. Proc. Natl Acad. Sci. USA 25, 405–416 (1939).
Szostak, J. W. & Blackburn, E. H. Cloning yeast telomeres on linear plasmid vectors. Cell 29, 245–255 (1982).
Shampay, J., Szostak, J. W. & Blackburn, E. H. DNA sequences of telomeres maintained in yeast. Nature 310, 154–157 (1984).
Blackburn, E. H. Switching and signaling at the telomere. Cell 106, 661–673 (2001).
Greider, C. W. & Blackburn, E. H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43, 405–413 (1985). In this seminal paper, telomerase activity and its ability to lengthen a (TTGGGG) n oligonucleotide is identified in Tetrahymena cell extracts.
Greider, C. W. & Blackburn, E. H. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell 51, 887–898 (1987).
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621 (1961).
Maser, R. S. & DePinho, R. A. Connecting chromosomes, crisis, and cancer. Science 297, 565–569 (2002).
Bodnar, A. G. et al. Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352 (1998). This seminal paper showed that reintroduction of telomerase into human retinal epithelial cells and fibroblasts prevents senescence and immortalizes human cells.
Counter, C. M. et al. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc. Natl Acad. Sci. USA 95, 14723–14728 (1998).
Cawthon, R. M., Smith, K. R., O'Brien, E., Sivatchenko, A. & Kerber, R. A. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361, 393–395 (2003).
Njajou, O. T. et al. Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition, a population-based cohort study. J. Gerontol. A 64, 860–864 (2009).
Atzmon, G. et al. Genetic variation in human telomerase is associated with telomere length in Ashkenazi centenarians. Proc. Natl Acad. Sci. USA 107 (suppl. 1), 1710–1717 (2010).
Epel, E. S. et al. Accelerated telomere shortening in response to life stress. Proc. Natl Acad. Sci. USA 101, 17312–17315 (2004).
Epel, E. S. et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology 31, 277–287 (2006).
Simon, N. M. et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol. Psychiatry 60, 432–435 (2006).
Passos, J. F. & von Zglinicki, T. Mitochondria, telomeres and cell senescence. Exp. Gerontol. 40, 466–472 (2005).
Oexle, K. & Zwirner, A. Advanced telomere shortening in respiratory chain disorders. Hum. Mol. Genet. 6, 905–908 (1997).
Kirwan, M. & Dokal, I. Dyskeratosis congenita, stem cells and telomeres. Biochim. Biophys. Acta 1792, 371–379 (2009).
Armanios, M. Y. et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N. Engl. J. Med. 356, 1317–1326 (2007).
Yamaguchi, H. et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N. Engl. J. Med. 352, 1413–1424 (2005).
Rudolph, K. L., Chang, S., Millard, M., Schreiber-Agus, N. & DePinho, R. A. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science 287, 1253–1258 (2000).
Blasco, M. A. et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91, 25–34 (1997). This paper follows from the McClintock hypotheses, providing experimental proof that telomerase activity has an essential role in maintaining telomeres and preventing end-to-end recombination, using telomerase knockout mice.
Farazi, P. A., Glickman, J., Horner, J. & Depinho, R. A. Cooperative interactions of p53 mutation, telomere dysfunction, and chronic liver damage in hepatocellular carcinoma progression. Cancer Res. 66, 4766–4773 (2006).
Lee, H. W. et al. Essential role of mouse telomerase in highly proliferative organs. Nature 392, 569–574 (1998).
Takai, H., Smogorzewska, A. & de Lange, T. DNA damage foci at dysfunctional telomeres. Curr. Biol. 13, 1549–1556 (2003).
Hande, M. P., Samper, E., Lansdorp, P. & Blasco, M. A. Telomere length dynamics and chromosomal instability in cells derived from telomerase null mice. J. Cell Biol. 144, 589–601 (1999).
Chang, S. et al. Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nature Genet. 36, 877–882 (2004).
Wong, K. K. et al. Telomere dysfunction and Atm deficiency compromises organ homeostasis and accelerates ageing. Nature 421, 643–648 (2003). This paper demonstrates that ATM deficiency and telomere dysfunction act together to impair stem-cell and progenitor-cell reserves and negatively affect cellular and whole-organism viability.
Rossi, D. J. et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447, 725–729 (2007). This paper shows that accumulated DNA damage in HSCs impairs their regenerative capacity, and it provides evidence that ageing-associated functional stem-cell decline is linked to DNA damage.
Choudhury, A. R. et al. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nature Genet. 39, 99–105 (2007).
Flores, I., Cayuela, M. L. & Blasco, M. A. Effects of telomerase and telomere length on epidermal stem cell behavior. Science 309, 1253–1256 (2005).
Ferron, S. et al. Telomere shortening and chromosomal instability abrogates proliferation of adult but not embryonic neural stem cells. Development 131, 4059–4070 (2004).
Sarin, K. Y. et al. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature 436, 1048–1052 (2005).
Maida, Y. et al. An RNA-dependent RNA polymerase formed by TERT and the RMRP RNA. Nature 461, 230–235 (2009).
Morales, M. et al. The Rad50S allele promotes ATM-dependent DNA damage responses and suppresses ATM deficiency: implications for the Mre11 complex as a DNA damage sensor. Genes Dev. 19, 3043–3054 (2005).
Inomata, K. et al. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell 137, 1088–1099 (2009).
Mostoslavsky, R. et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124, 315–329 (2006).
Michishita, E. et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature 452, 492–496 (2008).
Vousden, K. H. & Lane, D. P. p53 in health and disease. Nature Rev. Mol. Cell Biol. 8, 275–283 (2007).
Flores, I. & Blasco, M. A. A p53-dependent response limits epidermal stem cell functionality and organismal size in mice with short telomeres. PLoS ONE 4, e4934 (2009).
Artandi, S. E. et al. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406, 641–645 (2000).
Khoo, C. M., Carrasco, D. R., Bosenberg, M. W., Paik, J. H. & Depinho, R. A. Ink4a/Arf tumor suppressor does not modulate the degenerative conditions or tumor spectrum of the telomerase-deficient mouse. Proc. Natl Acad. Sci. USA 104, 3931–3936 (2007).
Chambers, S. M. et al. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 5, e201 (2007).
Donehower, L. A. & Lozano, G. 20 years studying p53 functions in genetically engineered mice. Nature Rev. Cancer 9, 831–841 (2009).
Matheu, A., Maraver, A. & Serrano, M. The Arf/p53 pathway in cancer and aging. Cancer Res. 68, 6031–6034 (2008).
Matheu, A. et al. Delayed ageing through damage protection by the Arf/p53 pathway. Nature 448, 375–379 (2007).
Mendrysa, S. M. et al. Tumor suppression and normal aging in mice with constitutively high p53 activity. Genes Dev. 20, 16–21 (2006).
Serrano, M. & Blasco, M. A. Cancer and ageing: convergent and divergent mechanisms. Nature Rev. Mol. Cell Biol. 8, 715–722 (2007).
Begus-Nahrmann, Y. et al. p53 deletion impairs clearance of chromosomal-instable stem cells in aging telomere-dysfunctional mice. Nature Genet. 41, 1138–1143 (2009).
Leri, A. et al. Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO J. 22, 131–139 (2003).
Sablina, A. A. et al. The antioxidant function of the p53 tumor suppressor. Nature Med. 11, 1306–1313 (2005).
Finkel, T., Deng, C. X. & Mostoslavsky, R. Recent progress in the biology and physiology of sirtuins. Nature 460, 587–591 (2009).
Trifunovic, A. et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423 (2004).
St- Pierre, J. et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127, 397–408 (2006). In this paper, the authors demonstrate that PGC-1 α is a strong positive regulator of the ROS defence system and that PGC-1 α deficiency leads to ROS-induced neurodegeneration.
Vianna, C. R. et al. Hypomorphic mutation of PGC-1β causes mitochondrial dysfunction and liver insulin resistance. Cell Metab. 4, 453–464 (2006).
Vijg, J. Aging of the Genome: The Dual Role of DNA in Life and Death (Oxford Univ. Press, 2007).
Ruzankina, Y. et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 1, 113–126 (2007).
Ito, K. et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 431, 997–1002 (2004).
Tomas- Loba, A. et al. Telomerase reverse transcriptase delays aging in cancer-resistant mice. Cell 135, 609–622 (2008).
Coviello- McLaughlin, G. M. & Prowse, K. R. Telomere length regulation during postnatal development and ageing in Mus spretus. Nucleic Acids Res. 25, 3051–3058 (1997).
Flores, I. et al. The longest telomeres: a general signature of adult stem cell compartments. Genes Dev. 22, 654–667 (2008).
Armanios, M. et al. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am. J. Hum. Genet. 85, 823–832 (2009).
Hao, L. Y. et al. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell 123, 1121–1131 (2005).
Martin, G. M. Genetic modulation of senescent phenotypes in Homo sapiens. Cell 120, 523–532 (2005).
Acknowledgements
We thank L. Chin, N. Sharpless, S. Artandi, F. Muller, V. Walsh, S. Colla, M. Ugolotti, M. Jaskelioff, D. Liu and A.-J. Chen for discussions and critical reading of the manuscript. A. Protopopov and E. Ivanova kindly provided the chromosomal and telomere analysis in Fig. 2. We apologize to the members of the scientific community whose work could not be cited owing to space limitations. Grant support for our work was provided by National Institutes of Health grants RO1CA84628 and U01 CA84313, US Department of Defense grant W81XWH-08-1-0133, and the Ellison Medical Foundation. R.A.D. is an American Cancer Society Research Professor and is supported by the Robert A. and Renée E. Belfer Foundation Institute for Innovative Cancer Science. E.S. was supported by the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Additional information
Reprints and permissions information is available at http://www.nature.com/reprints. The authors declare no competing financial interests. Correspondence should be addressed to the R.A.D. (ron_depinho@dfci.harvard.edu).
Rights and permissions
About this article
Cite this article
Sahin, E., DePinho, R. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 464, 520–528 (2010). https://doi.org/10.1038/nature08982
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08982
This article is cited by
-
Lack of telomerase reduces cancer incidence and increases lifespan of zebrafish tp53M214K mutants
Scientific Reports (2024)
-
Neuronal Stem Cells from Late-Onset Alzheimer Patients Show Altered Regulation of Sirtuin 1 Depending on Apolipoprotein E Indicating Disturbed Stem Cell Plasticity
Molecular Neurobiology (2024)
-
Induction of mitochondrial recycling reverts age-associated decline of the hematopoietic and immune systems
Nature Aging (2023)
-
Exploring the molecular mechanism underlying the psoriasis and T2D by using microarray data analysis
Scientific Reports (2023)
-
Prion infection modulates hematopoietic stem/progenitor cell fate through cell-autonomous and non-autonomous mechanisms
Leukemia (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.