Abstract
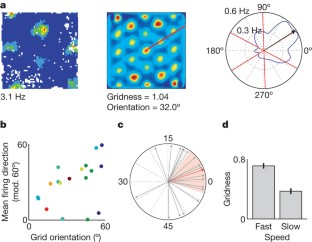
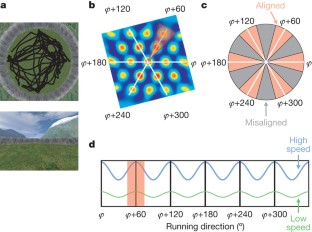
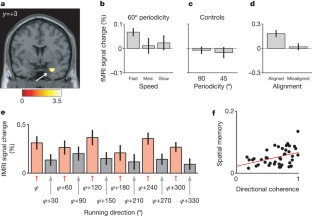
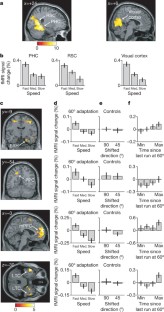
Grid cells in the entorhinal cortex of freely moving rats provide a strikingly periodic representation of self-location1 which is indicative of very specific computational mechanisms2,3,4. However, the existence of grid cells in humans and their distribution throughout the brain are unknown. Here we show that the preferred firing directions of directionally modulated grid cells in rat entorhinal cortex are aligned with the grids, and that the spatial organization of grid-cell firing is more strongly apparent at faster than slower running speeds. Because the grids are also aligned with each other1,5, we predicted a macroscopic signal visible to functional magnetic resonance imaging (fMRI) in humans. We then looked for this signal as participants explored a virtual reality environment, mimicking the rats’ foraging task: fMRI activation and adaptation showing a speed-modulated six-fold rotational symmetry in running direction. The signal was found in a network of entorhinal/subicular, posterior and medial parietal, lateral temporal and medial prefrontal areas. The effect was strongest in right entorhinal cortex, and the coherence of the directional signal across entorhinal cortex correlated with spatial memory performance. Our study illustrates the potential power of combining single-unit electrophysiology with fMRI in systems neuroscience. Our results provide evidence for grid-cell-like representations in humans, and implicate a specific type of neural representation in a network of regions which supports spatial cognition and also autobiographical memory.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hafting, T., Fyhn, M., Molden, S., Moser, M. B. & Moser, E. I. Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806 (2005)
McNaughton, B. L., Battaglia, F. P., Jensen, O., Moser, E. I. & Moser, M. B. Path integration and the neural basis of the ‘cognitive map’. Nature Rev. Neurosci. 7, 663–678 (2006)
Burgess, N., Barry, C. & O’Keefe, J. An oscillatory interference model of grid cell firing. Hippocampus 17, 801–812 (2007)
Hasselmo, M. E. A model of episodic memory: mental time travel along encoded trajectories using grid cells. Neurobiol. Learn. Mem. 92, 559–573 (2009)
Barry, C., Hayman, R., Burgess, N. & Jeffery, K. J. Experience-dependent rescaling of entorhinal grids. Nature Neurosci. 10, 682–684 (2007)
Sargolini, F. et al. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science 312, 758–762 (2006)
Boccara, C. N. et al. Grid cells in presubiculum and parasubiculum. FENS Abstr 4, 128.21 (2008)
Whitlock, J. R., Sutherland, R. J., Witter, M. P., Moser, M. B. & Moser, E. I. Navigating from hippocampus to parietal cortex. Proc. Natl Acad. Sci. USA 105, 14755–14762 (2008)
Logothetis, N. K. What we can do and what we cannot do with fMRI. Nature 453, 869–878 (2008)
Jeewajee, A., Barry, C., O’Keefe, J. & Burgess, N. Grid cells and theta as oscillatory interference: electrophysiological data from freely moving rats. Hippocampus 18, 1175–1185 (2008)
Doeller, C. F., King, J. A. & Burgess, N. Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proc. Natl Acad. Sci. USA 105, 5915–5920 (2008)
Fernandez, G., Brewer, J. B., Zhao, Z., Glover, G. H. & Gabrieli, J. D. Level of sustained entorhinal activity at study correlates with subsequent cued-recall performance: a functional magnetic resonance imaging study with high acquisition rate. Hippocampus 9, 35–44 (1999)
Olsen, R. K. et al. Performance-related sustained and anticipatory activity in human medial temporal lobe during delayed match-to-sample. J. Neurosci. 29, 11880–11890 (2009)
Epstein, R., Graham, K. S. & Downing, P. E. Viewpoint-specific scene representations in human parahippocampal cortex. Neuron 37, 865–876 (2003)
Grill-Spector, K., Henson, R. & Martin, A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn. Sci. 10, 14–23 (2006)
Park, S., Intraub, H., Yi, D. J., Widders, D. & Chun, M. M. Beyond the edges of a view: boundary extension in human scene-selective visual cortex. Neuron 54, 335–342 (2007)
Bakker, A., Kirwan, C. B., Miller, M. & Stark, C. E. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science 319, 1640–1642 (2008)
Ekstrom, A. D. et al. Cellular networks underlying human spatial navigation. Nature 425, 184–188 (2003)
Taube, J. S. Head direction cells and the neuropsychological basis for a sense of direction. Prog. Neurobiol. 55, 225–256 (1998)
Chen, L. L., Lin, L. H., Green, E. J., Barnes, C. A. & McNaughton, B. L. Head-direction cells in the rat posterior cortex. I. Anatomical distribution and behavioral modulation. Exp. Brain Res. 101, 8–23 (1994)
Byrne, P., Becker, S. & Burgess, N. Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol. Rev. 114, 340–375 (2007)
Wolbers, T., Wiener, J. M., Mallot, H. A. & Buchel, C. Differential recruitment of the hippocampus, medial prefrontal cortex, and the human motion complex during path integration in humans. J. Neurosci. 27, 9408–9416 (2007)
Maguire, E. A. Neuroimaging studies of autobiographical event memory. Phil. Trans. R. Soc. Lond. B 356, 1441–1451 (2001)
Schacter, D. L., Addis, D. R. & Buckner, R. L. Remembering the past to imagine the future: the prospective brain. Nature Rev. Neurosci. 8, 657–661 (2007)
O’Keefe, J. & Nadel, L. The Hippocampus as a Cognitive Map (Oxford Univ. Press, 1978)
Howard, M. W., Fotedar, M. S., Datey, A. V. & Hasselmo, M. E. The temporal context model in spatial navigation and relational learning: toward a common explanation of medial temporal lobe function across domains. Psychol. Rev. 112, 75–116 (2005)
Pastalkova, E., Itskov, V., Amarasingham, A. & Buzsaki, G. Internally generated cell assembly sequences in the rat hippocampus. Science 321, 1322–1327 (2008)
Gaffan, D. Scene-specific memory for objects: a model of episodic memory impairment in monkeys with fornix transection. J. Cogn. Neurosci. 6, 305–320 (1994)
Knierim, J. J. Neural representations of location outside the hippocampus. Learn. Mem. 13, 405–415 (2006)
Eichenbaum, H., Yonelinas, A. P. & Ranganath, C. The medial temporal lobe and recognition memory. Annu. Rev. Neurosci. 30, 123–152 (2007)
Recce, M. & O’Keefe, J. The tetrode: a new technique for multiunit extracellular recording. Soc. Neurosci. Abstr. 15, 1250 (1989)
Weiskopf, N., Hutton, C., Josephs, O. & Deichmann, R. Optimal EPI parameters for reduction of susceptibility-induced BOLD sensitivity losses: a whole-brain analysis at 3 T and 1.5 T. Neuroimage 33, 493–504 (2006)
Hutton, C. et al. Image distortion correction in fMRI: A quantitative evaluation. Neuroimage 16, 217–240 (2002)
Andersson, J. L., Hutton, C., Ashburner, J., Turner, R. & Friston, K. Modeling geometric deformations in EPI time series. Neuroimage 13, 903–919 (2001)
Ashburner, J. & Friston, K. J. Unified segmentation. Neuroimage 26, 839–851 (2005)
Büchel, C., Holmes, A. P., Rees, G. & Friston, K. J. Characterizing stimulus-response functions using nonlinear regressors in parametric fMRI experiments. Neuroimage 8, 140–148 (1998)
Fischl, B. et al. Predicting the location of entorhinal cortex from MRI. Neuroimage 47, 8–17 (2009)
Insausti, R., Tunon, T., Sobreviela, T., Insausti, A. M. & Gonzalo, L. M. The human entorhinal cortex: a cytoarchitectonic analysis. J. Comp. Neurol. 355, 171–198 (1995)
Acknowledgements
We acknowledge K. Jeffery and J. O’Keefe for providing help and facilities for single-unit recording; the Wellcome Trust Centre for Neuroimaging at UCL for providing help and scanning facilities; J. Krupic and R. Hayman for help with single-unit data collection; A. Jeewajee for help with analyses; J. King for help with virtual reality programming; and useful discussions with P. Dayan, K. Friston, U. Frith, C. Hall, A. Jeewajee, J. O’Keefe and M. Witter. This work was funded by the UK Medical Research Council and the European Union (SpaceBrain grant).
Author Contributions C.F.D., C.B. and N.B. jointly conceived and designed the experiments. C.F.D. performed the fMRI experiment and data analyses; C.B. performed the single-unit experiment and data analyses; N.B. gave direction on analyses; all authors discussed the analyses and results and contributed to writing the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-18 with Legends. (PDF 8238 kb)
Rights and permissions
About this article
Cite this article
Doeller, C., Barry, C. & Burgess, N. Evidence for grid cells in a human memory network. Nature 463, 657–661 (2010). https://doi.org/10.1038/nature08704
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08704
This article is cited by
-
A spatial transformation-based CAN model for information integration within grid cell modules
Cognitive Neurodynamics (2024)
-
Entorhinal grid-like codes and time-locked network dynamics track others navigating through space
Nature Communications (2023)
-
Task state representations in vmPFC mediate relevant and irrelevant value signals and their behavioral influence
Nature Communications (2023)
-
From cognitive maps to spatial schemas
Nature Reviews Neuroscience (2023)
-
Neural Correlates of Spatial Navigation in Primate Hippocampus
Neuroscience Bulletin (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.



