Abstract
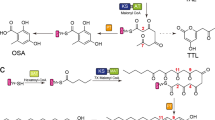
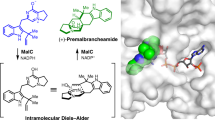
Polyketides are a class of natural products with diverse structures and biological activities. The structural variability of aromatic products of fungal nonreducing, multidomain iterative polyketide synthases (NR-PKS group of IPKSs) results from regiospecific cyclizations of reactive poly-β-keto intermediates1,2,3. How poly-β-keto species are synthesized and stabilized, how their chain lengths are determined, and, in particular, how specific cyclization patterns are controlled have been largely inaccessible and functionally unknown until recently4. A product template (PT) domain is responsible for controlling specific aldol cyclization and aromatization of these mature polyketide precursors, but the mechanistic basis is unknown. Here we present the 1.8 Å crystal structure and mutational studies of a dissected PT monodomain from PksA, the NR-PKS that initiates the biosynthesis of the potent hepatocarcinogen aflatoxin B1 in Aspergillus parasiticus. Despite having minimal sequence similarity to known enzymes, the structure displays a distinct ‘double hot dog’ (DHD) fold. Co-crystal structures with palmitate or a bicyclic substrate mimic illustrate that PT can bind both linear and bicyclic polyketides. Docking and mutagenesis studies reveal residues important for substrate binding and catalysis, and identify a phosphopantetheine localization channel and a deep two-part interior binding pocket and reaction chamber. Sequence similarity and extensive conservation of active site residues in PT domains suggest that the mechanistic insights gleaned from these studies will prove general for this class of IPKSs, and lay a foundation for defining the molecular rules controlling NR-PKS cyclization specificity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Staunton, J. & Weissman, K. J. Polyketide biosynthesis: a millennium review. Nat. Prod. Rep. 18, 380–416 (2001)
Thomas, R. A biosynthetic classification of fungal and Streptomycete fused-ring aromatic polyketides. ChemBioChem 2, 612–627 (2001)
Hertweck, C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. 48, 4688–4716 (2009)
Crawford, J. M. et al. Deconstruction of iterative multidomain polyketide synthase function. Science 320, 243–246 (2008)
Townsend, C. A. & Minto, R. E. in Comprehensive Natural Products 443–471 (Elsevier, 1999)
Udwary, D. W., Merski, M. & Townsend, C. A. Method for prediction of the locations of linker regions within large multifunctional proteins, and application to a type I polyketide synthase. J. Mol. Biol. 323, 585–598 (2002)
Crawford, J. M., Dancy, B. C. R., Hill, E. A., Udwary, D. W. & Townsend, C. A. Identification of a starter unit acyl-carrier protein transacylase domain in an iterative type I polyketide synthase. Proc. Natl Acad. Sci. USA 103, 16728–16733 (2006)
Dillon, S. C. & Bateman, A. The Hotdog fold: wrapping up a superfamily of thioesterases and dehydratases. BMC Bioinformatics 5, 109 (2004)
Maier, T., Leibundgut, M. & Ban, N. The crystal structure of a mammalian fatty acid synthase. Science 321, 1315–1322 (2008)
Keatinge-Clay, A. Crystal structure of the erythromycin polyketide synthase dehydratase. J. Mol. Biol. 384, 941–953 (2008)
Koski, K. M., Haapalainen, A. M., Hiltunen, J. K. & Glumoff, T. Crystal structure of 2-enoyl-CoA hydratase 2 from human peroxisomal multifunctional enzyme type 2. J. Mol. Biol. 345, 1157–1169 (2005)
Kimber, M. S. et al. The structure of (3R)-hydroxyacyl-acyl carrier protein dehydratase (FabZ) from Pseudomonas aeruginosa . J. Biol. Chem. 279, 52593–52602 (2004)
Hibbert, F. & Emsley, J. Hydrogen bonding and chemical reactivity. Adv. Phys. Org. Chem. 26, 255–379 (1991)
Mills, S. G. & Beak, P. Solvent effects on keto-enol equilibria: tests of quantitative models. J. Org. Chem. 50, 1216–1224 (1985)
Jordan, D. B., Zheng, Y. J., Lockett, B. A. & Basarab, G. S. Stereochemistry of the enolization of scytalone by scytalone dehydratase. Biochemistry 39, 2276–2282 (2000)
Bahnson, B. J., Anderson, V. E. & Petsko, G. A. Structural mechanism of enoyl-CoA hydratase: three atoms from a single water are added in either an E1cb stepwise or concerted fashion. Biochemistry 41, 2621–2629 (2002)
Sachdeva, S. et al. Separate entrance and exit portals for ligand traffic in Mycobacterium tuberculosis FabH. Chem. Biol. 15, 402–412 (2008)
Kroken, S., Glass, N. L., Taylor, J. W., Yoder, O. C. & Turgeon, B. G. Phylogenomic analysis of type I polyketide synthase genes in pathogenic and saprobic Ascomycetes . Proc. Natl Acad. Sci. USA 100, 15670–15675 (2003)
Ma, S. M. et al. Redirecting the cyclization steps of fungal polyketide synthase. J. Am. Chem. Soc. 130, 38–39 (2008)
Ames, B. D. et al. Crystal structure and functional analysis of tetracenomycin ARO/CYC: implications for cyclization specificity of aromatic polyketides. Proc. Natl Acad. Sci. USA 105, 5349–5354 (2008)
Verdonk, M. L., Cole, J. C., Hartshorn, M. J., Murray, C. W. & Taylor, R. D. Improved protein-ligand docking using GOLD. Proteins 52, 609–623 (2003)
Gabb, H. A., Jackson, R. M. & Sternberg, M. J. Modelling protein docking using shape complementarity, electrostatics and biochemical information. J. Mol. Biol. 272, 106–120 (1997)
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008)
Terwilliger, T. C. SOLVE and RESOLVE: automated structure solution, density modification and model building. J. Synchrotron Radiat. 11, 49–52 (2004)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Brünger, A. T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998)
Collaborative Computational Project, 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Schüttelkopf, A. W. & van Aalten, D. M. PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr. D 60, 1355–1363 (2004)
Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 (1993)
Ho, S. N., Hunt, H. D., Horton, R. M., Pullen, J. K. & Pease, L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77, 51–59 (1989)
Acknowledgements
We thank T. M. Harris for his gift of HC8. The work at Johns Hopkins was supported by the US National Institutes of Health grant ES001670 awarded to C.A.T. S.-C.T. is supported by the Pew Foundation. Portions of this research were carried out at the Stanford Synchrotron Radiation Laboratory (SSRL), a national user facility operated by Stanford University on behalf of the US Department of Energy, Office of Basic Energy Sciences. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under Contract No. DE-AC02-05CH11231. J.M.C. is currently a fellow supported by the Damon Runyon Cancer Research Foundation (DRG-2002-09, Harvard Medical School).
Author Contributions J.M.C. carried out biochemical experiments and provided recombinant proteins. A.L.V. carried out all mutational studies. T.P.K. assisted by O.K.-B. determined the PT X-ray crystal structures. E.A.H. prepared substrate and intermediate analogues for co-crystallization experiments. J.W.L. and T.P.K. conducted in silico docking studies. J.M.C., T.P.K. and J.W.L. analysed data and contributed to the writing of the paper, and J.W.L. refined the proposed PT mechanism. S.-C.T. and C.A.T. directed the research, provided funding and edited the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Information
This file contains a Supplementary Discussion, Supplementary Methods, Supplementary Tables S1-S3, Supplementary Figures S1-S6 with Legends and Supplementary References. (PDF 6194 kb)
Rights and permissions
About this article
Cite this article
Crawford, J., Korman, T., Labonte, J. et al. Structural basis for biosynthetic programming of fungal aromatic polyketide cyclization. Nature 461, 1139–1143 (2009). https://doi.org/10.1038/nature08475
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature08475
This article is cited by
-
Engineering of PKS Megaenzymes—A Promising Way to Biosynthesize High-Value Active Molecules
Topics in Catalysis (2022)
-
Saccharomyces cerevisiae as host for the recombinant production of polyketides and nonribosomal peptides
Microbial Cell Factories (2021)
-
A polyketoacyl-CoA thiolase-dependent pathway for the synthesis of polyketide backbones
Nature Catalysis (2020)
-
Docking analysis of hexanoic acid and quercetin with seven domains of polyketide synthase A provided insight into quercetin-mediated aflatoxin biosynthesis inhibition in Aspergillus flavus
3 Biotech (2019)
-
The plant hormone abscisic acid regulates the growth and metabolism of endophytic fungus Aspergillus nidulans
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.