Abstract
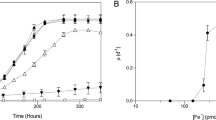
The discovery of ammonia oxidation by mesophilic and thermophilic Crenarchaeota and the widespread distribution of these organisms in marine and terrestrial environments indicated an important role for them in the global nitrogen cycle1,2,3,4,5,6,7. However, very little is known about their physiology or their contribution to nitrification8. Here we report oligotrophic ammonia oxidation kinetics and cellular characteristics of the mesophilic crenarchaeon ‘Candidatus Nitrosopumilus maritimus’ strain SCM1. Unlike characterized ammonia-oxidizing bacteria, SCM1 is adapted to life under extreme nutrient limitation, sustaining high specific oxidation rates at ammonium concentrations found in open oceans. Its half-saturation constant (Km = 133 nM total ammonium) and substrate threshold (≤10 nM) closely resemble kinetics of in situ nitrification in marine systems9,10 and directly link ammonia-oxidizing Archaea to oligotrophic nitrification. The remarkably high specific affinity for reduced nitrogen (68,700 l per g cells per h) of SCM1 suggests that Nitrosopumilus-like ammonia-oxidizing Archaea could successfully compete with heterotrophic bacterioplankton and phytoplankton. Together these findings support the hypothesis that nitrification is more prevalent in the marine nitrogen cycle than accounted for in current biogeochemical models11.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Schleper, C., Jurgens, G. & Jonuscheit, M. Genomic studies of uncultivated archaea. Nature Rev. Microbiol. 3, 479–488 (2005)
Francis, C. A., Roberts, K. J., Beman, J. M., Santoro, A. E. & Oakley, B. B. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl Acad. Sci. USA 102, 14683–14688 (2005)
Könneke, M. et al. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437, 543–546 (2005)
de la Torre, J. R., Walker, C. B., Ingalls, A. E., Könneke, M. & Stahl, D. A. Cultivation of a thermophilic ammonia oxidizing archaeon synthesizing crenarchaeol. Environ. Microbiol. 10, 810–818 (2008)
Wuchter, C. et al. Archaeal nitrification in the ocean. Proc. Natl Acad. Sci. USA 103, 12317–12322 (2006)
Hatzenpichler, R. et al. A moderately thermophilic ammonia-oxidizing crenarchaeote from a hot spring. Proc. Natl Acad. Sci. USA 105, 2134–2139 (2008)
Mincer, T. J. et al. Quantitative distribution of presumptive archaeal and bacterial nitrifiers in Monterey Bay and the North Pacific Subtropical Gyre. Environ. Microbiol. 9, 1162–1175 (2007)
Prosser, J. I. & Nicol, G. W. Relative contributions of archaea and bacteria to aerobic ammonia oxidation in the environment. Environ. Microbiol. 10, 2931–2941 (2008)
Hashimoto, L. K., Kaplan, W. A., Wofsy, S. C. & McElroy, M. B. Transformations of fixed nitrogen and N2O in the Cariaco Trench. Deep-Sea Res. 30, 575–590 (1983)
Olson, R. J. 15N tracer studies of the primary nitrite maximum. J. Mar. Res. 39, 203–226 (1981)
Yool, A., Martin, A. P., Fernandez, C. & Clark, D. R. The significance of nitrification for oceanic new production. Nature 447, 999–1002 (2007)
Gruber, N. & Galloway, J. N. An Earth-system perspective of the global nitrogen cycle. Nature 451, 293–296 (2008)
Prosser, J. I. in Advances in Microbial Physiology (eds Rose, A. H. & Tempest, D. W.) 125–181 (Academic, 1989)
Ward, B. B. in Nitrification (ed. Prosser, J. I.) 157–184 (IRL Press, 1986)
Bollmann, A., Bär-Gilissen, M.-J. & Laanbroek, H. J. Growth at low ammonium concentrations and starvation response as potential factors involved in niche differentiation among ammonia-oxidizing bacteria. Appl. Environ. Microbiol. 68, 4751–4757 (2002)
Agogué, H., Brink, M., Dinasquet, J. & Herndl, G. J. Major gradients in putatively nitrifying and non-nitrifying Archaea in the deep North Atlantic. Nature 456, 788–791 (2008)
Karner, M. B., DeLong, E. F. & Karl, D. M. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 409, 507–510 (2001)
Kirchman, D. L., Elifantz, H., Dittel, A. I., Malmstrom, R. R. & Cottrell, M. T. Standing stocks and activity of Archaea and Bacteria in the western Arctic Ocean. Limnol. Oceanogr. 52, 495–507 (2007)
Laanbroek, H. J. & Woldendorp, J. W. in Advances in Microbial Ecology (ed. Jones, J. G.) 275–304 (Plenum, 1995)
Leininger, S. et al. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442, 806–809 (2006)
Stark, J. M. & Firestone, M. K. Kinetic characteristics of ammonium-oxidizer communities in a California oak woodland-annual grassland. Soil Biol. Biochem. 28, 1307–1317 (1996)
Ward, B. B., Talbot, M. C. & Perry, M. J. Contributions of phytoplankton and nitrifying bacteria to ammonium and nitrite dynamics in coastal waters. Cont. Shelf Res. 3, 383–398 (1984)
Karl, D. M., Knauer, G. A., Martin, J. H. & Ward, B. B. Bacterial chemolithotrophy in the ocean is associated with sinking particles. Nature 309, 54–56 (1984)
Phillips, C. J., Smith, Z., Embley, T. M. & Prosser, J. I. Phylogenetic differences between particle-associated and planktonic ammonia-oxidizing bacteria of the β subdivision of the class Proteobacteria in the northwestern Mediterranean Sea. Appl. Environ. Microbiol. 65, 779–786 (1999)
Freitag, T. E., Chang, L. & Prosser, J. I. Changes in the community structure and activity of betaproteobacterial ammonia-oxidizing sediment bacteria along a freshwater-marine gradient. Environ. Microbiol. 8, 684–696 (2006)
Button, D. K., Robertson, B. R., Lepp, P. W. & Schmidt, T. M. A small, dilute-cytoplasm, high-affinity, novel bacterium isolated by extinction culture and having kinetic constants compatible with growth at ambient concentrations of dissolved nutrients in seawater. Appl. Environ. Microbiol. 64, 4467–4476 (1998)
Button, D. K. Nutrient uptake by microorganisms according to kinetic parameters from theory as related to cytoarchitecture. Microbiol. Mol. Biol. Rev. 62, 636–645 (1998)
Kirchman, D. L. The uptake of inorganic nutrients by heterotrophic bacteria. Microb. Ecol. 28, 255–271 (1994)
Eppley, R. W., Rogers, J. N. & McCarthy, J. J. Half-saturation constants for uptake of nitrate and ammonium by marine phytoplankton. Limnol. Oceanogr. 14, 912–920 (1969)
Ouverney, C. C. & Fuhrman, J. A. Marine planktonic archaea take up amino acids. Appl. Environ. Microbiol. 66, 4829–4833 (2000)
Berube, P. M., Samudrala, R. & Stahl, D. A. Transcription of all amoC copies is associated with recovery of Nitrosomonas europaea from ammonia starvation. J. Bacteriol. 189, 3935–3944 (2007)
Rotthauwe, J.-H., Witzel, K. P. & Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 63, 4704–4712 (1997)
Stickland, J. D. H. & Parsons, T. R. A Practical Handbook of Seawater Analysis (Fisheries Research Board of Canada, 1972)
Holmes, R. M., Aminot, A., Kérouel, R., Hooker, B. A. & Peterson, B. J. A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can. J. Fish. Aquat. Sci. 56, 1801–1809 (1999)
Lunau, M., Lemke, A., Walther, K., Martens-Habbena, W. & Simon, M. An improved method for counting bacteria from sediments and turbid environments by epifluorescence microscopy. Environ. Microbiol. 7, 961–968 (2005)
Gundersen, J. K., Ramsing, N. B. & Glud, R. N. Predicting the signal of O2 microsensors from physical dimensions, temperature, salinity, and O2 concentration. Limnol. Oceanogr. 43, 1932–1937 (1998)
Suzuki, I., Dular, U. & Kwok, S. C. Ammonia or ammonium ion as substrate for oxidation by Nitrosomonas europaea cells and extracts. J. Bacteriol. 120, 556–558 (1974)
Ward, B. B. Kinetic studies on ammonia and methane oxidation by Nitrosococcus oceanus . Arch. Microbiol. 147, 126–133 (1987)
Bollmann, A., Schmidt, I., Saunders, A. M. & Nicolaisen, M. H. Influence of starvation on potential ammonia-oxidizing activity and amoA mRNA levels of Nitrosospira briensis . Appl. Environ. Microbiol. 71, 1276–1282 (2005)
Button, D. K. Kinetics of nutrient-limited transport and microbial growth. Microbiol. Rev. 49, 270–297 (1985)
Eppley, R. W. & Renger, E. H. Nitrogen assimilation of an oceanic diatom in nitrogen-limited continuous culture. J. Phycol. 10, 15–23 (1974)
Glover, H. E. The relationship between inorganic nitrogen oxidation and organic-carbon production in batch and chemostat cultures of marine nitrifying bacteria. Arch. Microbiol. 142, 45–50 (1985)
Jiang, Q. Q. & Bakken, L. R. Comparison of Nitrosospira strains isolated from terrestrial environments. FEMS Microbiol. Ecol. 30, 171–186 (1999)
Keen, G. A. & Prosser, J. I. Steady state and transient growth of autotrophic nitrifying bacteria. Arch. Microbiol. 147, 73–79 (1987)
Loureiro, S. et al. The significance of organic nutrients in the nutrition of Pseudo-nitzschia delicatissima (Bacillariophyceae). J. Plankt. Res. 31, 399–410 (2009)
Reay, D. S., Nedwell, D. B., Priddle, J. & Ellis-Evans, J. C. Temperature dependence of inorganic nitrogen uptake: reduced affinity for nitrate at suboptimal temperatures in both algae and bacteria. Appl. Environ. Microbiol. 65, 2577–2584 (1999)
Stehr, G., Böttcher, B., Dittberner, P., Rath, G. & Koops, H. P. The ammonia-oxidizing nitrifying population of the River Elbe estuary. FEMS Microbiol. Ecol. 17, 177–186 (1995)
Suwa, Y., Imamura, Y., Suzuki, T., Tashiro, T. & Urushigawa, Y. Ammonia-oxidizing bacteria with different sensitivities to (NH4)2SO4 in activated sludges. Wat. Res. 28, 1523–1532 (1994)
Ward, B. B. Kinetics of ammonia oxidation by a marine nitrifying bacterium: methane as a substrate analogue. Microb. Ecol. 19, 211–225 (1990)
Watson, S. W. Characteristics of a marine nitrifying bacterium, Nitrosocystis oceanus sp. N. Limnol. Oceanogr. 10, R274–R289 (1965)
Clegg, S. L. & Whitfield, M. A chemical model of seawater including dissolved ammonia and the stoichiometric dissociation constant of ammonia in estuarine water and seawater from -2 to 40°C. Geochim. Cosmochim. Acta 59, 2403–2421 (1995)
Lee, S. & Fuhrman, J. A. Relationships between biovolume and biomass of naturally derived marine bacterioplankton. Appl. Environ. Microbiol. 53, 1298–1303 (1987)
Simon, M. & Azam, F. Protein content and protein synthesis rates of planktonic marine bacteria. Mar. Ecol. Prog. Ser. 51, 201–213 (1989)
Acknowledgements
We thank A. M. Gee for technical assistance and C. B. Walker, K. C. Costa, S. Flagan, D. K. Button, M. G. Klotz, L. Bakken, S. Sandfest, M. Könneke, K. L. Hillesland and L. H. Larsen for discussions. This work was supported by NSF award MCB-0604448 to D.A.S. and J.R.T. and by NSF award OCE-0623174 to A. E. Ingalls, D.A.S. and A. H. Devol.
Author Contributions W.M.-H., J.R.T. and D.A.S. designed research; W.M.-H., P.M.B. and H.U. performed research; W.M.-H., J.R.T. and D.A.S. analysed the data; and W.M.-H. and D.A.S. wrote the paper.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Information
This file contains Supplementary Notes 1-3. Supplementary References, and Supplementary Figures 1-3 with Legends. (PDF 619 kb)
Rights and permissions
About this article
Cite this article
Martens-Habbena, W., Berube, P., Urakawa, H. et al. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461, 976–979 (2009). https://doi.org/10.1038/nature08465
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08465
This article is cited by
-
Spent mushroom substrate as a substitute for chemical fertilizer changes N-cycling genes and reduces N2O emission in different textured soils
Biology and Fertility of Soils (2024)
-
Nitrogen metabolism pathways and functional microorganisms in typical karst wetlands
Environmental Science and Pollution Research (2024)
-
A unique subseafloor microbiosphere in the Mariana Trench driven by episodic sedimentation
Marine Life Science & Technology (2024)
-
Nitrification and beyond: metabolic versatility of ammonia oxidising archaea
The ISME Journal (2023)
-
Nitrogen and phosphorous acquisition strategies drive coexistence patterns among archaeal lineages in soil
The ISME Journal (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.