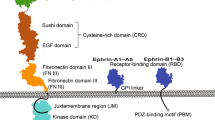
Abstract
The orphan receptor tyrosine kinase ErbB2 (also known as HER2 or Neu) transforms cells when overexpressed1, and it is an important therapeutic target in human cancer2,3. Structural studies4,5 have suggested that the oncogenic (and ligand-independent) signalling properties of ErbB2 result from the absence of a key intramolecular ‘tether’ in the extracellular region that autoinhibits other human ErbB receptors, including the epidermal growth factor (EGF) receptor6. Although ErbB2 is unique among the four human ErbB receptors6,7, here we show that it is the closest structural relative of the single EGF receptor family member in Drosophila melanogaster (dEGFR). Genetic and biochemical data show that dEGFR is tightly regulated by growth factor ligands8, yet a crystal structure shows that it, too, lacks the intramolecular tether seen in human EGFR, ErbB3 and ErbB4. Instead, a distinct set of autoinhibitory interdomain interactions hold unliganded dEGFR in an inactive state. All of these interactions are maintained (and even extended) in ErbB2, arguing against the suggestion that ErbB2 lacks autoinhibition. We therefore suggest that normal and pathogenic ErbB2 signalling may be regulated by ligands in the same way as dEGFR. Our findings have important implications for ErbB2 regulation in human cancer, and for developing therapeutic approaches that target novel aspects of this orphan receptor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Di Fiore, P. P. et al. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science 237, 178–182 (1987)
Hynes, N. E. & Lane, H. A. ERBB receptors and cancer: the complexity of targeted inhibitors. Nature Rev. Cancer 5, 341–354 (2005)
Moasser, M. M. Targeting the function of the HER2 oncogene in human cancer therapeutics. Oncogene 26, 6577–6592 (2007)
Cho, H. S. et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 421, 756–760 (2003)
Garrett, T. P. et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol. Cell 11, 495–505 (2003)
Burgess, A. W. et al. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol. Cell 12, 541–552 (2003)
Citri, A., Skaria, K. B. & Yarden, Y. The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp. Cell Res. 284, 54–65 (2003)
Shilo, B. Z. Regulating the dynamics of EGF receptor signaling in space and time. Development 132, 4017–4027 (2005)
Lemmon, M. A. Ligand-induced ErbB receptor dimerization. Exp. Cell Res. 315, 638–648 (2009)
Garrett, T. P. et al. Crystal structure of a truncated epidermal growth factor receptor extracellular domain bound to transforming growth factor α. Cell 110, 763–773 (2002)
Ogiso, H. et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell 110, 775–787 (2002)
Bouyain, S., Longo, P. A., Li, S., Ferguson, K. M. & Leahy, D. J. The extracellular region of ErbB4 adopts a tethered conformation in the absence of ligand. Proc. Natl Acad. Sci. USA 102, 15024–15029 (2005)
Klein, D. E., Nappi, V. M., Reeves, G. T., Shvartsman, S. Y. & Lemmon, M. A. Argos inhibits epidermal growth factor receptor signalling by ligand sequestration. Nature 430, 1040–1044 (2004)
Schweitzer, R., Shaharabany, M., Seger, R. & Shilo, B. Z. Secreted Spitz triggers the DER signaling pathway and is a limiting component in embryonic ventral ectoderm determination. Genes Dev. 9, 1518–1529 (1995)
Dawson, J. P., Bu, Z. & Lemmon, M. A. Ligand-induced structural transitions in ErbB receptor extracellular domains. Structure 15, 942–954 (2007)
Dawson, J. P. et al. Epidermal growth factor receptor dimerization and activation require ligand-induced conformational changes in the dimer interface. Mol. Cell. Biol. 25, 7734–7742 (2005)
Ferguson, K. M. et al. EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization. Mol. Cell 11, 507–517 (2003)
Mattoon, D., Klein, P., Lemmon, M. A., Lax, I. & Schlessinger, J. The tethered configuration of the EGF receptor extracellular domain exerts only a limited control of receptor function. Proc. Natl Acad. Sci. USA 101, 923–928 (2004)
Walker, F. et al. CR1/CR2 interactions modulate the functions of the cell surface epidermal growth factor receptor. J. Biol. Chem. 279, 22387–22398 (2004)
Cho, H. S. & Leahy, D. J. Structure of the extracellular region of HER3 reveals an interdomain tether. Science 297, 1330–1333 (2002)
Ferguson, K. M., Darling, P. J., Mohan, M. J., Macatee, T. L. & Lemmon, M. A. Extracellular domains drive homo- but not hetero-dimerization of erbB receptors. EMBO J. 19, 4632–4643 (2000)
Horan, T. et al. Binding of Neu differentiation factor with the extracellular domain of Her2 and Her3. J. Biol. Chem. 270, 24604–24608 (1995)
Penuel, E., Akita, R. W. & Sliwkowski, M. X. Identification of a region within the ErbB2/HER2 intracellular domain that is necessary for ligand-independent association. J. Biol. Chem. 277, 28468–28473 (2002)
Wehrman, T. S. et al. A system for quantifying dynamic protein interactions defines a role for Herceptin in modulating ErbB2 interactions. Proc. Natl Acad. Sci. USA 103, 19063–19068 (2006)
Berger, M. B., Mendrola, J. M. & Lemmon, M. A. ErbB3/HER3 does not homodimerize upon neuregulin binding at the cell surface. FEBS Lett. 569, 332–336 (2004)
Brennan, P. J., Kumagai, T., Berezov, A., Murali, R. & Greene, M. I. HER2/neu: mechanisms of dimerization/oligomerization. Oncogene 19, 6093–6101 (2000)
Carraway, K. L., Ramsauer, V. P., Haq, B. & Carothers Carraway, C. A. Cell signaling through membrane mucins. BioEssays 25, 66–71 (2003)
Miura, G. I. et al. Palmitoylation of the EGFR ligand Spitz by Rasp increases Spitz activity by restricting its diffusion. Dev. Cell 10, 167–176 (2006)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
CCP4 (Collaborative Computational Project Number 4). The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Klein, D. E., Stayrook, S. E., Shi, F., Narayan, K. & Lemmon, M. A. Structural basis for EGFR ligand sequestration by Argos. Nature 453, 1271–1275 (2008)
Svergun, D. I. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Crystallogr. 25, 495–503 (1992)
Svergun, D. I. Restoring low resolution structure of biological macromolecules from solution scattering using simulated annealing. Biophys. J. 76, 2879–2886 (1999)
Volkov, V. V. & Svergun, D. I. Uniqueness of ab initio shape determination in small-angle scattering. J. Appl. Crystallogr. 36, 860–864 (2003)
Wriggers, W. & Birmanns, S. Using Situs for flexible and rigid-body fitting of multiresolution single-molecule data. J. Struct. Biol. 133, 193–202 (2001)
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004)
Clifford, R. & Schupbach, T. Molecular analysis of the Drosophila EGF receptor homolog reveals that several genetically defined classes of alleles cluster in subdomains of the receptor protein. Genetics 137, 531–550 (1994)
Lesokhin, A. M., Yu, S. Y., Katz, J. & Baker, N. E. Several levels of EGF receptor signaling during photoreceptor specification in wild-type, Ellipse, and null mutant Drosophila . Dev. Biol. 205, 129–144 (1999)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
Brunger, A. T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998)
Winn, M. D., Isupov, M. N. & Murshudov, G. N. Use of TLS anisotropic displacements in macromolecular refinement. Acta Crystallogr. D 57, 122–133 (2001)
Lawrence, M. C. & Colman, P. M. Shape complementarity at protein/protein interfaces. J. Mol. Biol. 234, 946–950 (1993)
DeLano, W. L. The PyMOL Molecular Graphics System (DeLano Scientific, 2002)
Acknowledgements
We thank members of the Lemmon and Ferguson laboratories, K. Ferguson, G. Van Duyne, C. Abrams and J. Shorter for advice and comments on the manuscript; Z. Bu for assistance with collecting SAXS data; and R. Gillilan of MacCHESS for help with SAXS data collection and processing at CHESS beamline G1. Crystallographic results were obtained in research conducted at the GM/CA Collaborative Access Team at the Advanced Photon Source (APS), supported with funds from the National Cancer Institute and National Institute of General Medical Science. This study was supported by grants from the NIH (to M.A.L.). D.E.K. was supported by a Predoctoral fellowship from the US Army Breast Cancer Research Program. D.A. was supported by an NIH Postdoctoral Training Grant and a Postdoctoral Fellowship from the Damon Runyon Cancer Research Foundation.
Author Contributions D.A., D.E.K. and M.A.L. conceived and designed the project. D.E.K. established initial expression and purification procedures for s-dEGFR variants and relevant ligands. D.A. was responsible for designing s-dEGFR constructs used in this study and executed all biophysical studies, crystallization and data collection. D.A. also solved and refined the s-dEGFRΔV structure. D.A. and M.A.L. interpreted data and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures S1-S7 with Legends, Supplementary Tables 1-3 and Supplementary References. (PDF 1114 kb)
Rights and permissions
About this article
Cite this article
Alvarado, D., Klein, D. & Lemmon, M. ErbB2 resembles an autoinhibited invertebrate epidermal growth factor receptor. Nature 461, 287–291 (2009). https://doi.org/10.1038/nature08297
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08297
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.