Abstract
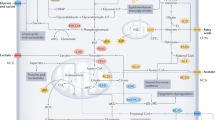
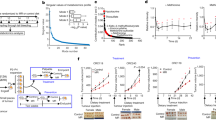
Dietary restriction delays the incidence and decreases the growth of various types of tumours, but the mechanisms underlying the sensitivity of tumours to food restriction remain unknown. Here we show that certain human cancer cell lines, when grown as tumour xenografts in mice, are highly sensitive to the anti-growth effects of dietary restriction, whereas others are resistant. Cancer cells that form dietary-restriction-resistant tumours carry mutations that cause constitutive activation of the phosphatidylinositol-3-kinase (PI3K) pathway and in culture proliferate in the absence of insulin or insulin-like growth factor 1. Substitution of an activated mutant allele of PI3K with wild-type PI3K in otherwise isogenic cancer cells, or the restoration of PTEN expression in a PTEN-null cancer cell line, is sufficient to convert a dietary-restriction-resistant tumour into one that is dietary-restriction-sensitive. Dietary restriction does not affect a PTEN-null mouse model of prostate cancer, but it significantly decreases tumour burden in a mouse model of lung cancer lacking constitutive PI3K signalling. Thus, the PI3K pathway is an important determinant of the sensitivity of tumours to dietary restriction, and activating mutations in the pathway may influence the response of cancers to dietary restriction-mimetic therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
30 April 2020
"An amendment to this paper has been published and can be accessed via a link at the top of the paper."
References
Moreschi, C. Beziehung zwischen Ernahrung und Tumorwachstum. Zeitschrift f. Immunitätsforsch. Originale 2, 651–675 (1909)
Rous, P. The influence of diet on transplanted and spontaneous tumors. J. Exp. Med. 20, 351–413 (1914)
McCay, C. M., Crowell, M. F. & Maynard, L. A. The effect of retarded growth upon the length of life span and upon the ultimate body size. J. Nutr. 10, 63–79 (1935)
Tannenbaum, A. & Silverstone, H. The influence of the degree of caloric restriction on the formation of skin tumors and hepatomas in mice. Cancer Res. 9, 724–727 (1949)
Tannenbaum, A. & Silverstone, H. Effect of limited food intake on survival of mice bearing spontaneous mammary carcinoma and on the incidence of lung metastases. Cancer Res. 13, 532–536 (1953)
Cheney, K. E. et al. Survival and disease patterns in C57BL/6J mice subjected to undernutrition. Exp. Gerontol. 15, 237–258 (1980)
Weindruch, R. & Walford, R. L. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science 215, 1415–1418 (1982)
Klurfeld, D. M., Welch, C. B., Davis, M. J. & Kritchevsky, D. Determination of degree of energy restriction necessary to reduce DMBA-induced mammary tumorigenesis in rats during the promotion phase. J. Nutr. 119, 286–291 (1989)
Zhu, Z., Haegele, A. D. & Thompson, H. J. Effect of caloric restriction on pre-malignant and malignant stages of mammary carcinogenesis. Carcinogenesis 18, 1007–1012 (1997)
Kritchevsky, D. Caloric restriction and cancer. J. Nutr. Sci. Vitaminol. (Tokyo) 47, 13–19 (2001)
Thompson, H. J., Zhu, Z. & Jiang, W. Dietary energy restriction in breast cancer prevention. J. Mammary Gland Biol. Neoplasia 8, 133–142 (2003)
Sell, C. Caloric restriction and insulin-like growth factors in aging and cancer. Horm. Metab. Res. 35, 705–711 (2003)
Cheney, K. E. et al. The effect of dietary restriction of varying duration on survival, tumor patterns, immune function, and body temperature in B10C3F1 female mice. J. Gerontol. 38, 420–430 (1983)
Pugh, T. D., Oberley, T. D. & Weindruch, R. Dietary intervention at middle age: caloric restriction but not dehydroepiandrosterone sulfate increases lifespan and lifetime cancer incidence in mice. Cancer Res. 59, 1642–1648 (1999)
Ruggeri, B. A., Klurfeld, D. M., Kritchevsky, D. & Furlanetto, R. W. Caloric restriction and 7,12-dimethylbenz(a)anthracene-induced mammary tumor growth in rats: alterations in circulating insulin, insulin-like growth factors I and II, and epidermal growth factor. Cancer Res. 49, 4130–4134 (1989)
Breese, C. R., Ingram, R. L. & Sonntag, W. E. Influence of age and long-term dietary restriction on plasma insulin-like growth factor-1 (IGF-1), IGF-1 gene expression, and IGF-1 binding proteins. J. Gerontol. 46, B180–B187 (1991)
Miller, F. R., Santner, S. J., Tait, L. & Dawson, P. J. MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ . J. Natl. Cancer Inst. 92, 1185–1186 (2000)
Manning, B. D. & Cantley, L. C. AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274 (2007)
Engelman, J. A., Luo, J. & Cantley, L. C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nature Rev. Genet. 7, 606–619 (2006)
Salmena, L., Carracedo, A. & Pandolfi, P. P. Tenets of PTEN tumor suppression. Cell 133, 403–414 (2008)
Samuels, Y. et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell 7, 561–573 (2005)
Kang, S., Bader, A. G. & Vogt, P. K. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc. Natl Acad. Sci. USA 102, 802–807 (2005)
Saal, L. H. et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 65, 2554–2559 (2005)
Zhao, J. J. et al. The oncogenic properties of mutant p110α and p110β phosphatidylinositol 3-kinases in human mammary epithelial cells. Proc. Natl Acad. Sci. USA 102, 18443–18448 (2005)
Samuels, Y. et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 304, 554 (2004)
Cancer Cell Line Project. <http://www.sanger.ac.uk/genetics/CGP/CellLines/> (1992)
Rodriguez-Viciana, P. et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 370, 527–532 (1994)
Gupta, S. et al. Binding of ras to phosphoinositide 3-kinase p110α is required for ras-driven tumorigenesis in mice. Cell 129, 957–968 (2007)
Calnan, D. R. & Brunet, A. The FoxO code. Oncogene 27, 2276–2288 (2008)
Radu, A., Neubauer, V., Akagi, T., Hanafusa, H. & Georgescu, M. M. PTEN induces cell cycle arrest by decreasing the level and nuclear localization of cyclin D1. Mol. Cell. Biol. 23, 6139–6149 (2003)
Wang, S. et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 4, 209–221 (2003)
Johnson, L. et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature 410, 1111–1116 (2001)
Ventura, A. et al. Restoration of p53 function leads to tumour regression in vivo . Nature 445, 661–665 (2007)
Fu, Z. & Tindall, D. J. FOXOs, cancer and regulation of apoptosis. Oncogene 27, 2312–2319 (2008)
Kim, D. H. et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110, 163–175 (2002)
Kalaany, N. Y. et al. LXRs regulate the balance between fat storage and oxidation. Cell Metab. 1, 231–244 (2005)
Wapnir, I. L., Wartenberg, D. E. & Greco, R. S. Three dimensional staging of breast cancer. Breast Cancer Res. Treat. 41, 15–19 (1996)
Carpenter, A. E. et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 7, R100 (2006)
Lamprecht, M. R., Sabatini, D. M. & Carpenter, A. E. CellProfiler: free, versatile software for automated biological image analysis. Biotechniques 42, 71–75 (2007)
Acknowledgements
We thank B. Vogelstein for providing the isogenic DLD-1 cell lines and M. M. Georgescu for the doxycycline-inducible U87-MG cell line; T. Jacks for the KRAS LA2; p53 LSL/WT mice and H. Wu for the Pb-Cre; PTEN L/L mice; F. Reinhardt for assistance with animal experiments; R. Bronson for histological analysis; the Histology Facility at the Koch Institute for Integrative Cancer Research and the Histology Core Laboratory at MIT for assistance with sectioning and immunohistochemistry; the Imaging Platform at the Broad Institute for assistance with image analysis; R. Weinberg, D. Guertin, Y. Chudnovsky and Y. Sancak for critical reading of the manuscript; and members of the Sabatini and Weinberg laboratories for support and discussions. This research is supported by the Alexander and Margaret Stewart Trust Award, the David H. Koch Cancer Research Award and National Institutes of Health grants R01 AI04389 and R01 CA129105. D.M.S. is an investigator of the Howard Hughes Medical Institute.
Author Contributions D.M.S. and N.Y.K. conceived the project and designed the experiments. N.Y.K. performed the experiments. The manuscript was written by N.Y.K. and edited by D.M.S.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains a Supplementary Discussion, Supplementary References and Supplementary Figures 1-4 with Legends (PDF 4035 kb)
Rights and permissions
About this article
Cite this article
Kalaany, N., Sabatini, D. Tumours with PI3K activation are resistant to dietary restriction. Nature 458, 725–731 (2009). https://doi.org/10.1038/nature07782
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07782
This article is cited by
-
Exercise and colorectal cancer: prevention and molecular mechanisms
Cancer Cell International (2022)
-
Developing dietary interventions as therapy for cancer
Nature Reviews Cancer (2022)
-
Increased O-GlcNAcylation promotes IGF-1 receptor/PhosphatidyI Inositol-3 kinase/Akt pathway in cervical cancer cells
Scientific Reports (2022)
-
Targeting lactate dehydrogenase a improves radiotherapy efficacy in non-small cell lung cancer: from bedside to bench
Journal of Translational Medicine (2021)
-
Molecular and cellular characterization of two patient-derived ductal carcinoma in situ (DCIS) cell lines, ETCC-006 and ETCC-010
BMC Cancer (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.