Abstract
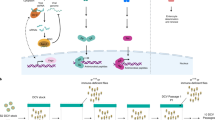
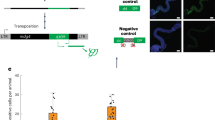
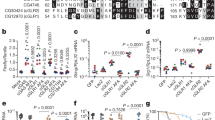
Multicellular organisms evolved sophisticated defence systems to confer protection against pathogens. An important characteristic of these immune systems is their ability to act both locally at the site of infection and at distal uninfected locations1,2,3,4. In insects, such as Drosophila melanogaster, RNA interference (RNAi) mediates antiviral immunity5,6,7. However, the antiviral RNAi defence in flies seems to be a local, cell-autonomous process, as flies are thought to be unable to generate a systemic RNAi response8. Here we show that a recently defined double-stranded RNA (dsRNA) uptake pathway9 is essential for effective antiviral RNAi immunity in adult flies. Mutant flies defective in this dsRNA uptake pathway were hypersensitive to infection with Drosophila C virus and Sindbis virus. Mortality in dsRNA-uptake-defective flies was accompanied by 100-to 105-fold increases in viral titres and higher levels of viral RNA. Furthermore, inoculating naked dsRNA into flies elicited a sequence-specific antiviral immune response that required an intact dsRNA uptake pathway. These findings suggest that spread of dsRNA to uninfected sites is essential for effective antiviral immunity. Notably, infection with green fluorescent protein (GFP)-tagged Sindbis virus suppressed expression of host-encoded GFP at a distal site. Thus, similar to protein-based immunity in vertebrates, the antiviral RNAi response in flies also relies on the systemic spread of a virus-specific immunity signal.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Baulcombe, D. RNA silencing in plants. Nature 431, 356–363 (2004)
Dorner, T. & Radbruch, A. Antibodies and B cell memory in viral immunity. Immunity 27, 384–392 (2007)
Roitt, I., Brostoff, J. & Male, D. Immunology (Mosby, 2001)
Voinnet, O. Non-cell autonomous RNA silencing. FEBS Lett. 579, 5858–5871 (2005)
Galiana-Arnoux, D., Dostert, C., Schneemann, A., Hoffmann, J. A. & Imler, J. L. Essential function in vivo for Dicer-2 in host defense against RNA viruses in Drosophila. Nature Immunol. 7, 590–597 (2006)
van Rij, R. P. et al. The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev. 20, 2985–2995 (2006)
Wang, X. H. et al. RNA interference directs innate immunity against viruses in adult Drosophila. Science 312, 452–454 (2006)
Roignant, J. Y. et al. Absence of transitive and systemic pathways allows cell-specific and isoform-specific RNAi in Drosophila. RNA 9, 299–308 (2003)
Saleh, M. C. et al. The endocytic pathway mediates cell entry of dsRNA to induce RNAi silencing. Nature Cell Biol. 8, 793–802 (2006)
Ulvila, J. et al. Double-stranded RNA is internalized by scavenger receptor-mediated endocytosis in Drosophila S2 cells. J. Biol. Chem. 281, 14370–14375 (2006)
Vaistij, F. E., Jones, L. & Baulcombe, D. C. Spreading of RNA targeting and DNA methylation in RNA silencing requires transcription of the target gene and a putative RNA-dependent RNA polymerase. Plant Cell 14, 857–867 (2002)
Sijen, T. et al. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell 107, 465–476 (2001)
Schubiger, M., Carre, C., Antoniewski, C. & Truman, J. W. Ligand-dependent de-repression via EcR/USP acts as a gate to coordinate the differentiation of sensory neurons in the Drosophila wing. Development 132, 5239–5248 (2005)
Lee, Y. S. et al. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell 117, 69–81 (2004)
Kennerdell, J. R. & Carthew, R. W. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell 95, 1017–1026 (1998)
Hoffmann, J. A. The immune response of Drosophila. Nature 426, 33–38 (2003)
Dostert, C. et al. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of Drosophila. Nature Immunol. 6, 946–953 (2005)
Porter, J. A., Minke, B. & Montell, C. Calmodulin binding to Drosophila NinaC required for termination of phototransduction. EMBO J. 14, 4450–4459 (1995)
Brennecke, J., Hipfner, D. R., Stark, A., Russell, R. B. & Cohen, S. M. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 113, 25–36 (2003)
Hahn, C. S., Hahn, Y. S., Braciale, T. J. & Rice, C. M. Infectious Sindbis virus transient expression vectors for studying antigen processing and presentation. Proc. Natl Acad. Sci. USA 89, 2679–2683 (1992)
Reed, L. J. & Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27, 493–497 (1938)
Okamura, K., Ishizuka, A., Siomi, H. & Siomi, M. C. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev. 18, 1655–1666 (2004)
Cherry, S. & Perrimon, N. Entry is a rate-limiting step for viral infection in a Drosophila melanogaster model of pathogenesis. Nature Immunol. 5, 81–87 (2004)
Goto, A., Blandin, S., Royet, J., Reichhart, J. M. & Levashina, E. A. Silencing of Toll pathway components by direct injection of double-stranded RNA into Drosophila adult flies. Nucleic Acids Res. 31, 6619–6623 (2003)
Acknowledgements
We are grateful to members of the Andino and the O’Farrell laboratories for discussion, technical support and advice on fly work. We thank J. Frydman, P. O’Farrell and M. Vignuzzi for discussions and comments on the manuscript. We also thank T. Cook for advice on the IR EcR eye phenotype, R. Carthew for providing the GMR>IR[white] and Dcr2 fly stocks and M. Siomi for the Ago2 fly stock. B.G. is a Manlio Cantarini fellow. B.B. is a Lebanese CNRSL Fellow. C.J. is a University of Paris VI and Ministère de la Recherche fellow. This work was financially supported by NIH grants AI40085 and AI064738 to R.A., the Institut Pasteur to M.-C.S. and C.A., and CNRS, ANR and ARC grants to C.A.
Author Contributions M.-C.S., M.T. and R.P.v.R. performed dsRNA inoculations and virus infections, normal and reverse northern blotting, western blotting, survival curves, obtained fluorescent images, and prepared and analysed mutant flies. B.G. and V.G. examined systemic spread of dsRNA. The genetic and phenotypic analyses of transgenic flies expressing RNA hairpins were designed and carried out by B.B., C.J. and C.A. M.-C.S. and R.A. designed the experiments, discussed the interpretation of the results and co-wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figures
This file contains Supplementary Figures S1-S6 with Legends (PDF 1388 kb)
Rights and permissions
About this article
Cite this article
Saleh, MC., Tassetto, M., van Rij, R. et al. Antiviral immunity in Drosophila requires systemic RNA interference spread. Nature 458, 346–350 (2009). https://doi.org/10.1038/nature07712
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07712
This article is cited by
-
Aedes aegypti Argonaute 2 controls arbovirus infection and host mortality
Nature Communications (2023)
-
Shrimp genome sequence contains independent clusters of ancient and current Endogenous Viral Elements (EVE) of the parvovirus IHHNV
BMC Genomics (2022)
-
Plant and animal small RNA communications between cells and organisms
Nature Reviews Molecular Cell Biology (2022)
-
Adaptive immunity or evolutionary adaptation? Transgenerational immune systems at the crossroads
Biology & Philosophy (2022)
-
First transcriptome of the Neotropical pest Euschistus heros (Hemiptera: Pentatomidae) with dissection of its siRNA machinery
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.