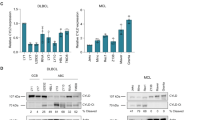
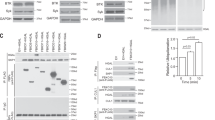
Abstract
The transcription factor NF-κB is required for lymphocyte activation and proliferation as well as the survival of certain lymphoma types1,2. Antigen receptor stimulation assembles an NF-κB activating platform containing the scaffold protein CARMA1 (also called CARD11), the adaptor BCL10 and the paracaspase MALT1 (the CBM complex), linked to the inhibitor of NF-κB kinase complex3,4,5,6,7,8,9,10,11,12, but signal transduction is not fully understood1. We conducted parallel screens involving a mass spectrometry analysis of CARMA1 binding partners and an RNA interference screen for growth inhibition of the CBM-dependent ‘activated B-cell-like’ (ABC) subtype of diffuse large B-cell lymphoma (DLBCL)12. Here we report that both screens identified casein kinase 1α (CK1α) as a bifunctional regulator of NF-κB. CK1α dynamically associates with the CBM complex on T-cell-receptor (TCR) engagement to participate in cytokine production and lymphocyte proliferation. However, CK1α kinase activity has a contrasting role by subsequently promoting the phosphorylation and inactivation of CARMA1. CK1α has thus a dual ‘gating’ function which first promotes and then terminates receptor-induced NF-κB. ABC DLBCL cells required CK1α for constitutive NF-κB activity, indicating that CK1α functions as a conditionally essential malignancy gene—a member of a new class of potential cancer therapeutic targets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hacker, H. & Karin, M. Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006, re13 (2006)
Schulze-Luehrmann, J. & Ghosh, S. Antigen-receptor signaling to nuclear factor κB. Immunity 25, 701–715 (2006)
Egawa, T. et al. Requirement for CARMA1 in antigen receptor-induced NF-κB activation and lymphocyte proliferation. Curr. Biol. 13, 1252–1258 (2003)
Gaide, O. et al. CARMA1 is a critical lipid raft-associated regulator of TCR-induced NF-κB activation. Nature Immunol. 3, 836–843 (2002)
Hara, H. et al. The MAGUK family protein CARD11 is essential for lymphocyte activation. Immunity 18, 763–775 (2003)
Jun, J. E. & Goodnow, C. C. Scaffolding of antigen receptors for immunogenic versus tolerogenic signaling. Nature Immunol. 4, 1057–1064 (2003)
Newton, K. & Dixit, V. M. Mice lacking the CARD of CARMA1 exhibit defective B lymphocyte development and impaired proliferation of their B and T lymphocytes. Curr. Biol. 13, 1247–1251 (2003)
Ruefli-Brasse, A. A., French, D. M. & Dixit, V. M. Regulation of NF-κB-dependent lymphocyte activation and development by paracaspase. Science 302, 1581–1584 (2003)
Ruland, J. et al. Bcl10 is a positive regulator of antigen receptor-induced activation of NF-κB and neural tube closure. Cell 104, 33–42 (2001)
Ruland, J., Duncan, G. S., Wakeham, A. & Mak, T. W. Differential requirement for Malt1 in T and B cell antigen receptor signaling. Immunity 19, 749–758 (2003)
Wang, D. et al. A requirement for CARMA1 in TCR-induced NF-κB activation. Nature Immunol. 3, 830–835 (2002)
Ngo, V. N. et al. A loss-of-function RNA interference screen for molecular targets in cancer. Nature 441, 106–110 (2006)
Price, M. A. CKI, there’s more than one: casein kinase I family members in Wnt and Hedgehog signaling. Genes Dev. 20, 399–410 (2006)
Oeckinghaus, A. et al. Malt1 ubiquitination triggers NF-κB signaling upon T-cell activation. EMBO J. 26, 4634–4645 (2007)
Bidere, N., Snow, A. L., Sakai, K., Zheng, L. & Lenardo, M. J. Caspase-8 regulation by direct interaction with TRAF6 in T cell receptor-induced NF-κB activation. Curr. Biol. 16, 1666–1671 (2006)
Matsumoto, R. et al. Phosphorylation of CARMA1 plays a critical role in T cell receptor-mediated NF-κB activation. Immunity 23, 575–585 (2005)
Sommer, K. et al. Phosphorylation of the CARMA1 linker controls NF-κB activation. Immunity 23, 561–574 (2005)
Shinohara, H. et al. PKC β regulates BCR-mediated IKK activation by facilitating the interaction between TAK1 and CARMA1. J. Exp. Med. 202, 1423–1431 (2005)
Shambharkar, P. B. et al. Phosphorylation and ubiquitination of the IκB kinase complex by two distinct signaling pathways. EMBO J. 26, 1794–1805 (2007)
Davidson, G. et al. Casein kinase 1 γ couples Wnt receptor activation to cytoplasmic signal transduction. Nature 438, 867–872 (2005)
Peters, J. M., McKay, R. M., McKay, J. P. & Graff, J. M. Casein kinase I transduces Wnt signals. Nature 401, 345–350 (1999)
Wegener, E. et al. Essential role for IκB kinase β in remodeling Carma1-Bcl10-Malt1 complexes upon T cell activation. Mol. Cell 23, 13–23 (2006)
Alizadeh, A. A. et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403, 503–511 (2000)
Rosenwald, A. et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N. Engl. J. Med. 346, 1937–1947 (2002)
Davis, R. E., Brown, K. D., Siebenlist, U. & Staudt, L. M. Constitutive nuclear factor κB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J. Exp. Med. 194, 1861–1874 (2001)
Lenz, G. et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science 319, 1676–1679 (2008)
Liu, C. et al. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108, 837–847 (2002)
Shaffer, A. L. et al. IRF4 addiction in multiple myeloma. Nature 454, 226–231 (2008)
Solimini, N. L., Luo, J. & Elledge, S. J. Non-oncogene addiction and the stress phenotype of cancer cells. Cell 130, 986–988 (2007)
Su, H. et al. Requirement for caspase-8 in NF-κB activation by antigen receptor. Science 307, 1465–1468 (2005)
Wan, F. et al. Ribosomal protein S3: a KH domain subunit in NF-κB complexes that mediates selective gene regulation. Cell 131, 927–939 (2007)
Acknowledgements
This work was supported by the Intramural Research Program of the NIH, NIAID, NCI, NIDDK and by the Agence Nationale de la Recherche (ANR). We thank M.-T. Auffredou for technical assistance; X. Lin and J. Gavard for reagents; R. Germain, R. Schwartz, U. Siebenlist, P. Schwartzberg, J. Bosco de Oliveira, L. Yu, D. Baltimore, P. Sharp, H. Varmus and A. Snow for discussions and comments; and S. Porcella and the DNA sequencing core facility of the Rocky Mountain Laboratories, NIAID.
Author Contributions N.B., V.N.N., J.L., C.C., L.Z., F.W., R.E.D., G.L., L.M.S. and M.J.L. carried out experimental design/discussion; N.B., V.N.N., J.L., C.C., L.Z., F.W., R.E.D., G.L., D.E.A., D.A., A.V., K.S., J.Z., Z.M. and T.D.V. carried out preparation and performance of experiments; N.B., V.N.N., L.M.S. and M.J.L. carried out manuscript preparation. All authors discussed the results and commented on the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Figures
This file contains Supplementary Figures S1-S11 with Legends (PDF 1732 kb)
Rights and permissions
About this article
Cite this article
Bidère, N., Ngo, V., Lee, J. et al. Casein kinase 1α governs antigen-receptor-induced NF-κB activation and human lymphoma cell survival. Nature 458, 92–96 (2009). https://doi.org/10.1038/nature07613
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07613
This article is cited by
-
Casein kinase 1α mediates eryptosis: a review
Apoptosis (2023)
-
A20 and ABIN-1 cooperate in balancing CBM complex-triggered NF-κB signaling in activated T cells
Cellular and Molecular Life Sciences (2022)
-
Targeting the CK1α/CBX4 axis for metastasis in osteosarcoma
Nature Communications (2020)
-
Prosurvival autophagy is regulated by protein kinase CK1 alpha in multiple myeloma
Cell Death Discovery (2019)
-
CARD–BCL-10–MALT1 signalling in protective and pathological immunity
Nature Reviews Immunology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.