Abstract
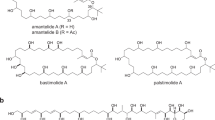
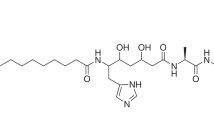
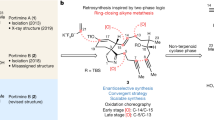
Each year, there are many cases of seafood poisoning in humans worldwide1. Among the various toxins isolated that contribute to these poisonings2,3, the chlorosulpholipids are particularly intriguing because of their structural and stereochemical complexity4,5,6,7,8,9,10,11,12. The mechanism of biological activity remains unknown and, although chlorosulpholipids are associated with membranes in the organisms from which they are isolated, little is understood about their role within biological membranes. The lack of availability of the natural products has impaired more in-depth biochemical studies. So far, none of the chlorosulpholipids have been obtained from total synthesis, and efficient routes to their synthesis would be desirable for the preparation of material for pharmacological characterization and proper evaluation of the risk to human health. Despite the notable advances in the science of organic synthesis, reliable methods for stereoselective construction of polychlorinated acyclic substrates are lacking, although some preliminary investigations have appeared13,14,15. Here we report the synthesis of a chlorosulpholipid cytotoxin, leading to confirmation of the proposed structure and the discovery of unanticipated reactivity of polychlorinated hydrocarbons. The concise synthetic approach should enable the preparation of material in sufficient quantities to facilitate biological studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Ahmed, F. E. Naturally occurring seafood toxins. J. Toxicol. Toxin Rev. 10, 263–287 (1991)
Ciminiello, P. & Fattorusso, E. Shellfish toxins – Chemical studies on Northern Adriatic mussels. Eur. J. Org. Chem. 2004, 2533–2551 (2004)
Yasumoto, T. & Murata, M. Marine toxins. Chem. Rev. 93, 1897–1909 (1993)
Elovson, J. & Vagelos, P. R. A new class of lipids: Chlorosulfolipids. Proc. Natl Acad. Sci. USA 62, 957–963 (1969)
Elovson, J. & Vagelos, P. R. Structure of the major species of chlorosulfolipid from Ochromonas danica. 2,2,11,13,15,16-hexachloro-N-docosane 1,14-disulfate. Biochemistry 9, 3110–3126 (1970)
Haines, T. H. Halogen- and sulfur-containing lipids of Ochromonas . Annu. Rev. Microbiol. 27, 403–411 (1973)
Mercer, E. I. & Davies, C. L. Chlorosulpholipids of Tribonema aequale . Phytochemistry 13, 1607–1610 (1974)
Mercer, E. I. & Davies, C. L. Chlorosulpholipids in algae. Phytochemistry 14, 1545–1548 (1975)
Chen, J. L., Proteau, P. J., Roberts, M. A. & Gerwick, W. H. Structure of malhamensilipin A, an inhibitor of protein tyrosine kinase, from the cultured chrysophyte Poterioochromonas malhamensis . J. Nat. Prod. 57, 524–527 (1994)
Ciminiello, P. et al. Structural elucidation of a new cytotoxin isolated from mussels of the Adriatic Sea. J. Org. Chem. 66, 578–582 (2001)
Ciminiello, P. et al. Structure and stereochemistry of a new cytotoxic polychlorinated sulfolipid from Adriatic shellfish. J. Am. Chem. Soc. 124, 13114–13120 (2002)
Ciminiello, P. et al. A new cytotoxic polychlorinated sulfolipid from contaminated Adriatic mussels. Tetrahedron 60, 7093–7098 (2004)
Shibuya, G. M., Kanady, J. S. & Vanderwal, C. D. Stereoselective dichlorination of allylic alcohol derivatives to access key stereochemical arrays of the chlorosulfolipids. J. Am. Chem. Soc. 130, 12514–12518 (2008)
Yoshimitsu, T., Fukumoto, N. & Tanaka, T. Enantiocontrolled synthesis of polychlorinated hydrocarbon motifs: A nucleophilic multiple chlorination process revisited. J. Org. Chem. 74, 696–702 (2009)
Hunter, L., O’Hagan, D. & Slawin, A. M. Z. Enantioselective synthesis of an all-syn four vicinal fluorine motif. J. Am. Chem. Soc. 128, 16422–16423 (2006)
Nakata, T. Total synthesis of marine polycyclic ethers. Chem. Rev. 105, 4314–4347 (2005)
Kang, S. H., Kang, S. Y., Lee, H.-S. & Buglass, A. J. Total synthesis of natural tert-alkylamino hydroxy carboxylic acids. Chem. Rev. 105, 4537–4558 (2005)
Matsumori, N., Kaneno, D., Murata, M., Nakamura, H. & Tachibana, K. Stereochemical determination of acyclic structures based on carbon-proton spin-coupling constants. A method of configuration analysis for natural products. J. Org. Chem. 64, 866–876 (1999)
Schlama, T., Gabriel, K., Gouverneur, V. & Mioskowski, C. Tetraethylammonium trichloride: A versatile reagent for chlorinations and oxidations. Angew. Chem. Int. Edn Engl. 36, 2342–2344 (1997)
Cha, J. K., Christ, W. J. & Kishi, Y. On stereochemistry of osmium tetraoxide oxidation of allylic alcohol systems – Empirical rule. Tetrahedron 40, 2247–2255 (1984)
Takai, K., Nitta, K. & Utimoto, K. Simple and selective method for RCHO → (E)-RCH = CHX conversion by means of a CHX3-CrCl2 system. J. Am. Chem. Soc. 108, 7408–7410 (1986)
Kozminski, W. & Nanz, D. HECADE: HMQC- and HSQC-based 2D NMR experiments for accurate and sensitive determination of heteronuclear coupling constants from E.COSY-type cross peaks. J. Magn. Reson. 124, 383–392 (1997)
Petersen, P. E. et al. Solvents of low nucleophilicity. IX. Inductive and participation effects in carbonium ion reactions in acetic, formic, and trifluoroacetic acid. J. Am. Chem. Soc. 89, 5902–5911 (1967)
Petersen, P. E., Indelicato, J. M. & Bonazza, B. R. Halogen participation in the protonation of 5-halo-epoxides with trifluoroacetic acid. Tetrahedr. Lett. 12, 13–16 (1971)
MacCoss, R. N., Balskus, E. P. & Ley, S. V. A sequential tetra-n-propylammonium perruthenate (TPAP)-Wittig oxidation olefination protocol. Tetrahedr. Lett. 44, 7779–7781 (2003)
Paddon-Row, M. N., Rondan, N. G. & Houk, K. N. Staggered models for asymmetric induction: Attack trajectories and conformations of allylic bonds from ab initio transition structures of addition reactions. J. Am. Chem. Soc. 104, 7162–7166 (1982)
Pangborn, A. B., Giardello, M. A., Grubbs, R. H., Rosen, R. K. & Timmers, F. J. Safe and convenient procedure for solvent purification. Organometallics 15, 1518–1520 (1996)
Acknowledgements
We thank M.-O. Ebert for acquisition and discussion of the NMR data needed for J-based configuration analysis as well as W. B. Schweizer for the X-ray crystallographic analysis. C.N. thanks the Stiftung Stipendien-Fonds des Verbandes der Chemischen Industrie for a Kekulé-Fellowship. This research was supported by the Swiss National Science Foundation and ETH Zürich. We are grateful for support for our program from F. Hoffmann-La Roche, Eli Lilly and Boehringer Ingelheim.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary crystallographic data for this paper has been deposited at the Cambridge Crystallographic Data Centre under deposition numbers CCDC 711103 and CCDC 711104. These data can be obtained free of charge from http://www.ccdc.cam.ac.uk/data_request/cif.
Supplementary information
Supplementary Information
This file contains Supplementary Methods and Supplementary Data. The operating frequencies of some of the NMR spectrometers were corrected on 4th Feb 2009. (PDF 1119 kb)
Rights and permissions
About this article
Cite this article
Nilewski, C., Geisser, R. & Carreira, E. Total synthesis of a chlorosulpholipid cytotoxin associated with seafood poisoning. Nature 457, 573–576 (2009). https://doi.org/10.1038/nature07734
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature07734
This article is cited by
-
Urea group-directed organocatalytic asymmetric versatile dihalogenation of alkenes and alkynes
Nature Catalysis (2021)
-
Catalytic, stereospecific syn-dichlorination of alkenes
Nature Chemistry (2015)
-
Chlorine lends a helping hand
Nature (2009)
-
Seafood surprise
Nature Chemistry (2009)
-
Our choice from the recent literature
Nature Chemistry (2009)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.