Abstract
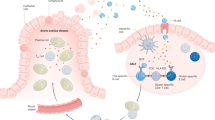
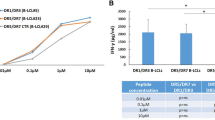
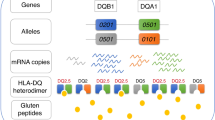
Major histocompatibility complex (MHC) class II alleles HLA-DQ8 and the mouse homologue I-Ag7 lacking a canonical aspartic acid residue at position β57 are associated with coeliac disease1,2 and type I diabetes3,4. However, the role of this single polymorphism in disease initiation and progression remains poorly understood. The lack of Asp 57 creates a positively charged P9 pocket, which confers a preference for negatively charged peptides. Gluten lacks such peptides, but tissue transglutaminase (TG2) introduces negatively charged residues at defined positions into gluten T-cell epitopes by deamidating specific glutamine residues5,6 on the basis of their spacing to proline residues7. The commonly accepted model, proposing that HLA-DQ8 simply favours binding of negatively charged peptides, does not take into account the fact that TG2 requires inflammation for activation8 and that T-cell responses against native gluten peptides are found9,10, particularly in children11. Here we show that β57 polymorphism promotes the recruitment of T-cell receptors bearing a negative signature charge in the complementary determining region 3β (CDR3β) during the response against native gluten peptides presented by HLA-DQ8 in coeliac disease. These T cells showed a crossreactive and heteroclitic (stronger) response to deamidated gluten peptides. Furthermore, gluten peptide deamidation extended the T-cell-receptor repertoire by relieving the requirement for a charged residue in CDR3β. Thus, the lack of a negative charge at position β57 in MHC class II was met by negatively charged residues in the T-cell receptor or in the peptide, the combination of which might explain the role of HLA-DQ8 in amplifying the T-cell response against dietary gluten.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sollid, L. M. et al. Evidence for a primary association of celiac disease to a particular HLA-DQ α/β heterodimer. J. Exp. Med. 169, 345–350 (1989)
Spurkland, A., Sollid, L. M., Polanco, I., Vartdal, F. & Thorsby, E. HLA-DR and -DQ genotypes of celiac disease patients serologically typed to be non-DR3 or non-DR5/7. Hum. Immunol. 35, 188–192 (1992)
Acha-Orbea, H. & McDevitt, H. O. The first domain of the nonobese diabetic mouse class II I-A β chain is unique. Proc. Natl Acad. Sci. USA 84, 2435–2439 (1987)
Hattori, M. et al. The NOD mouse: recessive diabetogenic gene in the major histocompatibility complex. Science 231, 733–735 (1986)
Molberg, O. et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nature Med. 4, 713–717 (1998)
van de Wal, Y. et al. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J. Immunol. 161, 1585–1588 (1998)
Vader, L. W. et al. Specificity of tissue transglutaminase explains cereal toxicity in celiac disease. J. Exp. Med. 195, 643–649 (2002)
Siegel, M. et al. Extracellular transglutaminase 2 is catalytically inactive, but is transiently activated upon tissue injury. PLoS ONE 3, e1861 (2008)
Henderson, K. N. et al. A structural and immunological basis for the role of human leukocyte antigen DQ8 in celiac disease. Immunity 27, 23–34 (2007)
van de Wal, Y. et al. Small intestinal T cells of celiac disease patients recognize a natural pepsin fragment of gliadin. Proc. Natl Acad. Sci. USA 95, 10050–10054 (1998)
Vader, W. et al. The gluten response in children with celiac disease is directed toward multiple gliadin and glutenin peptides. Gastroenterology 122, 1729–1737 (2002)
Jabri, B. & Sollid, L. M. Mechanisms of disease: immunopathogenesis of celiac disease. Nature Clin. Pract. Gastroenterol. Hepatol. 3, 516–525 (2006)
Lundin, K. E. et al. Gliadin-specific, HLA-DQ (α1*0501, β1*0201) restricted T cells isolated from the small intestinal mucosa of celiac disease patients. J. Exp. Med. 178, 187–196 (1993)
Lundin, K. E., Scott, H., Fausa, O., Thorsby, E. & Sollid, L. M. T cells from the small intestinal mucosa of a FR4, DQ7/DR4, DQ8 celiac disease patient preferentially recognize gliadin when presented by DQ8. Hum. Immunol. 41, 285–291 (1994)
Black, K. E., Murray, J. A. & David, C. S. HLA-DQ determines the response to exogenous wheat proteins: a model of gluten sensitivity in transgenic knockout mice. J. Immunol. 169, 5595–5600 (2002)
Moustakas, A. K. et al. Structure of celiac disease-associated HLA-DQ8 and non-associated HLA-DQ9 alleles in complex with two disease-specific epitopes. Int. Immunol. 12, 1157–1166 (2000)
Solinger, A. M., Ultee, M. E., Margoliash, E. & Schwartz, R. H. T-lymphocyte response to cytochrome c. I. Demonstration of a T-cell heteroclitic proliferative response and identification of a topographic antigenic determinant on pigeon cytochrome c whose immune recognition requires two complementing major histocompatibility complex-linked immune response genes. J. Exp. Med. 150, 830–848 (1979)
Bankovich, A. J., Girvin, A. T., Moesta, A. K. & Garcia, K. C. Peptide register shifting within the MHC groove: theory becomes reality. Mol. Immunol. 40, 1033–1039 (2004)
Baker, F. J., Lee, M., Chien, Y. H. & Davis, M. M. Restricted islet-cell reactive T cell repertoire of early pancreatic islet infiltrates in NOD mice. Proc. Natl Acad. Sci. USA 99, 9374–9379 (2002)
Yang, Y., Charlton, B., Shimada, A., Dal Canto, R. & Fathman, C. G. Monoclonal T cells identified in early NOD islet infiltrates. Immunity 4, 189–194 (1996)
Chao, C. C., Sytwu, H. K., Chen, E. L., Toma, J. & McDevitt, H. O. The role of MHC class II molecules in susceptibility to type I diabetes: identification of peptide epitopes and characterization of the T cell repertoire. Proc. Natl Acad. Sci. USA 96, 9299–9304 (1999)
Yu, B., Gauthier, L., Hausmann, D. H. & Wucherpfennig, K. W. Binding of conserved islet peptides by human and murine MHC class II molecules associated with susceptibility to type I diabetes. Eur. J. Immunol. 30, 2497–2506 (2000)
Ploski, R., Ek, J., Thorsby, E. & Sollid, L. M. On the HLA-DQ (α1*0501, β1*0201)-associated susceptibility in celiac disease: a possible gene dosage effect of DQB1*0201. Tissue Antigens 41, 173–177 (1993)
Vader, W. et al. The HLA-DQ2 gene dose effect in celiac disease is directly related to the magnitude and breadth of gluten-specific T cell responses. Proc. Natl Acad. Sci. USA 100, 12390–12395 (2003)
Quinn, A. et al. T cells to a dominant epitope of GAD65 express a public CDR3 motif. Int. Immunol. 18, 967–979 (2006)
Bendelac, A. et al. CD1 recognition by mouse NK1+ T lymphocytes. Science 268, 863–865 (1995)
Casanova, J. L., Romero, P., Widmann, C., Kourilsky, P. & Maryanski, J. L. T cell receptor genes in a series of class I major histocompatibility complex-restricted cytotoxic T lymphocyte clones specific for a Plasmodium berghei nonapeptide: implications for T cell allelic exclusion and antigen-specific repertoire. J. Exp. Med. 174, 1371–1383 (1991)
Arden, B., Clark, S. P., Kabelitz, D. & Mak, T. W. Mouse T cell receptor variable gene segment families. Immunogenetics 42, 501–530 (1995)
Blank, U., Boitel, B., Mege, D., Ermonval, M. & Acuto, O. Analysis of tetanus toxin peptide/DR recognition by human T cell receptors reconstituted into a murine T cell hybridoma. Eur. J. Immunol. 23, 3057–3065 (1993)
Scott, C. A., Garcia, K. C., Carbone, F. R., Wilson, I. A. & Teyton, L. Role of chain pairing for the production of functional soluble IA major histocompatibility complex class II molecules. J. Exp. Med. 183, 2087–2095 (1996)
Acknowledgements
We thank S. Sadegh-Nasseri for advice and discussion, and A. Bendelac and M. Mush for critical reading of the manuscript. This work was supported by the Digestive Disease Research Core Center of the University of Chicago (DK42086), NIH DK67180, DK55037, the Celiac Disease Consortium (F.K.), EU MC-RTN 512385 (M.W.), the Research Council of Norway (L.S.) and the University of Oslo (S.T.).
Author Contributions Z.H. executed most of the studies, participated in conceptual development and in the preparation of the manuscript; A.W., A.M., C.C., S.A.C., K.Y. provided technical assistance and input into data analysis. M.W., S.T., L.M.S. and F.K. generated and characterized human T-cell clones. C.S.D. and J.A.M. provided humanized HLA-DQ8 transgenic mice. F.K. and L.M.S. reviewed the manuscript. L.T. provided input into conceptual development and experimental design. B.J. developed the concept, supervised all investigations and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-3 with Legends, Supplementary Tables 1-3 and Supplementary Methods. (PDF 995 kb)
Rights and permissions
About this article
Cite this article
Hovhannisyan, Z., Weiss, A., Martin, A. et al. The role of HLA-DQ8 β57 polymorphism in the anti-gluten T-cell response in coeliac disease. Nature 456, 534–538 (2008). https://doi.org/10.1038/nature07524
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature07524
This article is cited by
-
RETRACTED ARTICLE: Mouse models of intestinal inflammation and cancer
Archives of Toxicology (2016)
-
T Cell Epitopes and Post-Translationally Modified Epitopes in Type 1 Diabetes
Current Diabetes Reports (2015)
-
How T cells taste gluten in celiac disease
Nature Structural & Molecular Biology (2014)
-
Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis
Nature Genetics (2013)
-
Animal models to study gluten sensitivity
Seminars in Immunopathology (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.