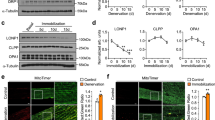
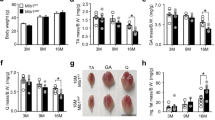
Abstract
Many neuromuscular conditions are characterized by an exaggerated exercise-induced fatigue response that is disproportionate to activity level. This fatigue is not necessarily correlated with greater central or peripheral fatigue in patients1, and some patients experience severe fatigue without any demonstrable somatic disease2. Except in myopathies that are due to specific metabolic defects, the mechanism underlying this type of fatigue remains unknown2. With no treatment available, this form of inactivity is a major determinant of disability3. Here we show, using mouse models, that this exaggerated fatigue response is distinct from a loss in specific force production by muscle, and that sarcolemma-localized signalling by neuronal nitric oxide synthase (nNOS) in skeletal muscle is required to maintain activity after mild exercise. We show that nNOS-null mice do not have muscle pathology and have no loss of muscle-specific force after exercise but do display this exaggerated fatigue response to mild exercise. In mouse models of nNOS mislocalization from the sarcolemma, prolonged inactivity was only relieved by pharmacologically enhancing the cGMP signal that results from muscle nNOS activation during the nitric oxide signalling response to mild exercise. Our findings suggest that the mechanism underlying the exaggerated fatigue response to mild exercise is a lack of contraction-induced signalling from sarcolemma-localized nNOS, which decreases cGMP-mediated vasomodulation in the vessels that supply active muscle after mild exercise. Sarcolemmal nNOS staining was decreased in patient biopsies from a large number of distinct myopathies, suggesting a common mechanism of fatigue. Our results suggest that patients with an exaggerated fatigue response to mild exercise would show clinical improvement in response to treatment strategies aimed at improving exercise-induced signalling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schillings, M. L. et al. Experienced and physiological fatigue in neuromuscular disorders. Clin. Neurophysiol. 118, 292–300 (2007)
Zwarts, M. J., Bleijenberg, G. & van Engelen, B. G. Clinical neurophysiology of fatigue. Clin. Neurophysiol doi: 10.1016/j.clinph.2007.09.126 (2007)
Kalkman, J. S., Schillings, M. L., Zwarts, M. J., van Engelen, B. G. & Bleijenberg, G. The development of a model of fatigue in neuromuscular disorders: A longitudinal study. J. Psychosom. Res. 62, 571–579 (2007)
Radley, H. G., De Luca, A., Lynch, G. S. & Grounds, M. D. Duchenne muscular dystrophy: focus on pharmaceutical and nutritional interventions. Int. J. Biochem. Cell Biol. 39, 469–477 (2007)
Duclos, F. et al. Progressive muscular dystrophy in α-sarcoglycan-deficient mice. J. Cell Biol. 142, 1461–1471 (1998)
Ozawa, E., Mizuno, Y., Hagiwara, Y., Sasaoka, T. & Yoshida, M. Molecular and cell biology of the sarcoglycan complex. Muscle Nerve 32, 563–576 (2005)
Consolino, C. M. et al. Muscles of mice deficient in α-sarcoglycan maintain large masses and near control force values throughout the life span. Physiol. Genomics 22, 244–256 (2005)
Yokoyama, T., Lisi, T. L., Moore, S. A. & Sluka, K. A. Muscle fatigue increases the probability of developing hyperalgesia in mice. J. Pain 8, 692–699 (2007)
Harper, S. Q. et al. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nature Med. 8, 253–261 (2002)
Imamura, M., Mochizuki, Y., Engvall, E. & Takeda, S. I. ε-Sarcoglycan compensates for lack of α-sarcoglycan in a mouse model of limb-girdle muscular dystrophy. Hum. Mol. Genet. 14, 775–783 (2005)
Phillips, B. A. & Mastaglia, F. L. Exercise therapy in patients with myopathy. Curr. Opin. Neurol. 13, 547–552 (2000)
Torelli, S. et al. Absence of neuronal nitric oxide synthase (nNOS) as a pathological marker for the diagnosis of Becker muscular dystrophy with rod domain deletions. Neuropathol. Appl. Neurobiol. 30, 540–545 (2004)
Judge, L. M., Haraguchiln, M. & Chamberlain, J. S. Dissecting the signaling and mechanical functions of the dystrophin–glycoprotein complex. J. Cell Sci. 119, 1537–1546 (2006)
Thomas, G. D., Shaul, P. W., Yuhanna, I. S., Froehner, S. C. & Adams, M. E. Vasomodulation by skeletal muscle-derived nitric oxide requires α-syntrophin-mediated sarcolemmal localization of neuronal nitric oxide synthase. Circ. Res. 92, 554–560 (2003)
Chao, D. S., Silvagno, F. & Bredt, D. S. Muscular dystrophy in mdx mice despite lack of neuronal nitric oxide synthase. J. Neurochem. 71, 784–789 (1998)
Crosbie, R. H. et al. mdx muscle pathology is independent of nNOS perturbation. Hum. Mol. Genet. 7, 823–829 (1998)
Suzuki, N. et al. NO production results in suspension-induced muscle atrophy through dislocation of neuronal NOS. J. Clin. Invest. 117, 2468–2476 (2007)
Asai, A. et al. Primary role of functional ischemia, quantitative evidence for the two-hit mechanism, and phosphodiesterase-5 inhibitor therapy in mouse muscular dystrophy. PLoS ONE 2, e806 (2007)
Huang, P. L. et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 377, 239–242 (1995)
Thomas, G. D. et al. Impaired metabolic modulation of α-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc. Natl Acad. Sci. USA 95, 15090–15095 (1998)
Bloom, T. J. Age-related alterations in cyclic nucleotide phosphodiesterase activity in dystrophic mouse leg muscle. Can. J. Physiol. Pharmacol. 83, 1055–1060 (2005)
Bloom, T. J. Cyclic nucleotide phosphodiesterase isozymes expressed in mouse skeletal muscle. Can. J. Physiol. Pharmacol. 80, 1132–1135 (2002)
Kass, D. A., Champion, H. C. & Beavo, J. A. Phosphodiesterase type 5: expanding roles in cardiovascular regulation. Circ. Res. 101, 1084–1095 (2007)
Persson, J., Ekelund, U. & Grande, P. O. Endogenous nitric oxide reduces microvascular permeability and tissue oedema during exercise in cat skeletal muscle. J. Vasc. Res. 40, 538–546 (2003)
Marden, F. A., Connolly, A. M., Siegel, M. J. & Rubin, D. A. Compositional analysis of muscle in boys with Duchenne muscular dystrophy using MR imaging. Skeletal Radiol. 34, 140–148 (2005)
Acknowledgements
We thank M. Anderson and M. Henry for comments, and M. M. Kilburg, K. Uppal, B. J. Steinmann and S. Watkins and members of the Campbell laboratory for scientific contributions. This work was supported in part by a Paul D. Wellstone Muscular Dystrophy Cooperative Research Center Grant. Y.M.K. was supported by grants from the University of Iowa Cardiovascular Interdisciplinary Research/ National Research Service Award (NRSA) Fellowship, from an individual NRSA Fellowship from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, from the National Institutes of Health (NIH), and from a Senator Paul D. Wellstone Fellowship. E.P.R. was supported by a Muscular Dystrophy Association Development Grant. R.M.W. was supported by the NIH. K.P.C. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Additional Methods, Supplementary Figures S1-S9 with Legends, Supplementary Table 1, Supplementary Methods and Supplementary References (PDF 1723 kb)
Movie file S1a
Movie file S1a shows Pre-exercise activity of a C57BL/10 wild-type mouse (MOV 6787 kb)
Movie file S1b
Movie file S1b shows Pre-exercise activity of an mdx mouse (MOV 6763 kb)
Movie file S1c
Movie file S1c shows Pre-exercise activity of a C57BL/6 wild-type mouse (MOV 6729 kb)
Movie file 1d
Movie file 1d shows Pre-exercise activity of an Sgca-null mouse (MOV 6831 kb)
Movie file 2a
Movie file 2a shows Post-exercise activity of a C57BL/10 wild-type mouse. (MOV 6728 kb)
Movie file 2b
Movie file 2b shows Post-exercise activity of an mdx mouse (MOV 6765 kb)
Movie file 2c
Movie file 2c shows Post-exercise activity of a C57BL/6 mouse wild-type mouse (MOV 6777 kb)
Movie file 2d
Movie file 2d shows Post-exercise activity of an Sgca-null mouse (MOV 6947 kb)
Rights and permissions
About this article
Cite this article
Kobayashi, Y., Rader, E., Crawford, R. et al. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature 456, 511–515 (2008). https://doi.org/10.1038/nature07414
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07414
This article is cited by
-
N-terminal titin fragment: a non-invasive, pharmacodynamic biomarker for microdystrophin efficacy
Skeletal Muscle (2024)
-
iPSCs ameliorate hypoxia-induced autophagy and atrophy in C2C12 myotubes via the AMPK/ULK1 pathway
Biological Research (2023)
-
A video game based hand grip system for measuring muscle force in children
Journal of NeuroEngineering and Rehabilitation (2021)
-
Therapeutic aspects of cell signaling and communication in Duchenne muscular dystrophy
Cellular and Molecular Life Sciences (2021)
-
Adapted physical activity and therapeutic exercise in late-onset Pompe disease (LOPD): a two-step rehabilitative approach
Neurological Sciences (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.