Abstract
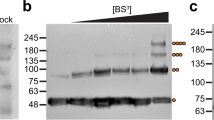
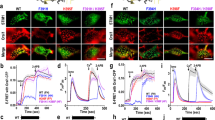
Ca2+-release-activated Ca2+ (CRAC) channels underlie sustained Ca2+ signalling in lymphocytes and numerous other cells after Ca2+ liberation from the endoplasmic reticulum (ER). RNA interference screening approaches identified two proteins, Stim1,2 and Orai3,4,5, that together form the molecular basis for CRAC channel activity6,7. Stim senses depletion of the ER Ca2+ store and physically relays this information by translocating from the ER to junctions adjacent to the plasma membrane1,8,9, and Orai embodies the pore of the plasma membrane calcium channel10,11,12. A close interaction between Stim and Orai, identified by co-immunoprecipitation12 and by Förster resonance energy transfer13, is involved in the opening of the Ca2+ channel formed by Orai subunits. Most ion channels are multimers of pore-forming subunits surrounding a central channel, which are preassembled in the ER and transported in their final stoichiometry to the plasma membrane. Here we show, by biochemical analysis after cross-linking in cell lysates and intact cells and by using non-denaturing gel electrophoresis without cross-linking, that Orai is predominantly a dimer in the plasma membrane under resting conditions. Moreover, single-molecule imaging of green fluorescent protein (GFP)-tagged Orai expressed in Xenopus oocytes showed predominantly two-step photobleaching, again consistent with a dimeric basal state. In contrast, co-expression of GFP-tagged Orai with the carboxy terminus of Stim as a cytosolic protein to activate the Orai channel without inducing Ca2+ store depletion or clustering of Orai into punctae yielded mostly four-step photobleaching, consistent with a tetrameric stoichiometry of the active Orai channel. Interaction with the C terminus of Stim thus induces Orai dimers to dimerize, forming tetramers that constitute the Ca2+-selective pore. This represents a new mechanism in which assembly and activation of the functional ion channel are mediated by the same triggering molecule.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Liou, J. et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 15, 1235–1241 (2005)
Roos, J. et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 169, 435–445 (2005)
Feske, S. et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441, 179–185 (2006)
Zhang, S. L. et al. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc. Natl Acad. Sci. USA 103, 9357–9362 (2006)
Vig, M. et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312, 1220–1223 (2006)
Cahalan, M. D. et al. Molecular basis of the CRAC channel. Cell Calcium 42, 133–144 (2007)
Lewis, R. S. The molecular choreography of a store-operated calcium channel. Nature 446, 284–287 (2007)
Zhang, S. L. et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437, 902–905 (2005)
Wu, M. M., Buchanan, J., Luik, R. M. & Lewis, R. S. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J. Cell Biol. 174, 803–813 (2006)
Prakriya, M. et al. Orai1 is an essential pore subunit of the CRAC channel. Nature 443, 230–233 (2006)
Vig, M. et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr. Biol. 16, 2073–2079 (2006)
Yeromin, A. V. et al. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature 443, 226–229 (2006)
Muik, M. et al. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J. Biol. Chem. 283, 8014–8022 (2008)
Gwack, Y. et al. Biochemical and functional characterization of Orai proteins. J. Biol. Chem. 282, 16232–16243 (2007)
Mignen, O., Thompson, J. L. & Shuttleworth, T. J. Orai1 subunit stoichiometry of the mammalian CRAC channel pore. J. Physiol. (Lond.) 586, 419–425 (2008)
Hoenderop, J. G. et al. Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J. 22, 776–785 (2003)
Kedei, N. et al. Analysis of the native quaternary structure of vanilloid receptor 1. J. Biol. Chem. 276, 28613–28619 (2001)
Raab-Graham, K. F. & Vandenberg, C. A. Tetrameric subunit structure of the native brain inwardly rectifying potassium channel Kir 2.2. J. Biol. Chem. 273, 19699–19707 (1998)
Ramjeesingh, M., Huan, L. J., Garami, E. & Bear, C. E. Novel method for evaluation of the oligomeric structure of membrane proteins. Biochem. J. 342, 119–123 (1999)
Huang, G. N. et al. STIM1 carboxyl-terminus activates native SOC, I crac and TRPC1 channels. Nature Cell Biol. 8, 1003–1010 (2006)
Zhang, S. L. et al. Store-dependent and -independent modes regulating CRAC channel activity of human Orai1 and Orai3. J. Biol. Chem. 283, 17662–17671 (2008)
Yeromin, A. V., Roos, J., Stauderman, K. A. & Cahalan, M. D. A store-operated calcium channel in Drosophila S2 cells. J. Gen. Physiol. 123, 167–182 (2004)
Ulbrich, M. H. & Isacoff, E. Y. Subunit counting in membrane-bound proteins. Nature Methods 4, 319–321 (2007)
Ji, W. et al. Functional stoichiometry of the unitary calcium-release-activated calcium channel. Proc. Natl Acad. Sci. USA doi: 10.1073/pnas.0806499105 (29 August 2008)
Zacharias, D. A., Violin, J. D., Newton, A. C. & Tsien, R. Y. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296, 913–916 (2002)
Newbolt, A. et al. Membrane topology of an ATP-gated ion channel (P2X receptor). J. Biol. Chem. 273, 15177–15182 (1998)
Nielsen, P. A. et al. Molecular cloning, functional expression, and tissue distribution of a novel human gap junction-forming protein, connexin-31.9. Interaction with zona occludens protein-1. J. Biol. Chem. 277, 38272–38283 (2002)
Aschrafi, A., Sadtler, S., Niculescu, C., Rettinger, J. & Schmalzing, G. Trimeric architecture of homomeric P2X2 and heteromeric P2X1+2 receptor subtypes. J. Mol. Biol. 342, 333–343 (2004)
Demuro, A. & Parker, I. Optical single-channel recording: imaging Ca2+ flux through individual ion channels with high temporal and spatial resolution. J. Biomed. Opt. 10, 11002 (2005)
Parker, I., Gundersen, C. B. & Miledi, R. A transient inward current elicited by hyperpolarization during serotonin activation in Xenopus oocytes. Proc. R. Soc. Lond. B 223, 279–292 (1985)
Dargan, S. L., Demuro, A. & Parker, I. Imaging Ca2+ signals in Xenopus oocytes. Methods Mol. Biol. 322, 103–119 (2006)
Acknowledgements
We thank L. Forrest for assistance in cell culture; T. Holmes and S. Leverrier for discussion during the course of the study; A. Amcheslavsky, A. Froger, K. Kalman and K. Cahalan for help with oocytes; W. Jiang for assistance in some of the co-immunoprecipitation experiments; M. Ramjeesingh for help with the PFO–PAGE technique; F.-A. Rassendren for the P2X2 construct; and E. Isacoff and M. Ulbrich for discussion and help during a pilot photobleaching study. This work was supported by grants from the National Institutes of Health (M.D.C. and I.P.) and by a fellowship from the George E. Hewitt Foundation (A.P.).
Author Contributions A.P. designed and performed cDNA and cRNA constructs, cell biology and all biochemical experiments. A.D. performed all experiments in oocytes and data analysis of single-molecule photobleaching. A.V.Y. was responsible for patch-clamp experiments and analysis. S.L.Z. made the C-Stim construct, performed Ca2+ imaging experiments and collaborated on co-immunoprecipitation experiments. O.S. provided general technical assistance. I.P. supervised single-molecule experiments on oocytes. M.D.C. provided advice and overall direction, and supervised project planning and execution. A.P., I.P. and M.D.C. wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Table 1, Supplementary Figures 1-8 with Legends and Supplementary Video Legends 1 and 2. (PDF 2386 kb)
Supplementary Video 1
This file contains Video 1. (MOV 1185 kb)
Supplementary Video 2
This file contains Video 2. (MOV 7605 kb)
Rights and permissions
About this article
Cite this article
Penna, A., Demuro, A., Yeromin, A. et al. The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers. Nature 456, 116–120 (2008). https://doi.org/10.1038/nature07338
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07338
This article is cited by
-
STIM1 in tumor cell death: angel or devil?
Cell Death Discovery (2023)
-
Endoplasmic reticulum in oocytes: spatiotemporal distribution and function
Journal of Assisted Reproduction and Genetics (2023)
-
Role of vitamin D and calcium signaling in epidermal wound healing
Journal of Endocrinological Investigation (2022)
-
Beta-Glycerophosphate-Induced ORAI1 Expression and Store Operated Ca2+ Entry in Megakaryocytes
Scientific Reports (2020)
-
Decreased Na+/K+ ATPase Expression and Depolarized Cell Membrane in Neurons Differentiated from Chorea-Acanthocytosis Patients
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.