Abstract
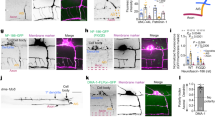
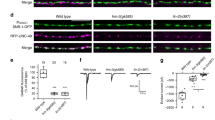
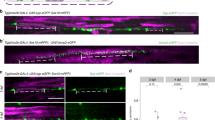
Polarity is an essential feature of many cell types, including neurons that receive information from local inputs within their dendrites and propagate nerve impulses to distant targets through a single axon. It is generally believed that intrinsic structural differences between axons and dendrites dictate the polarized localization of axonal and dendritic proteins1. However, whether extracellular cues also instruct this process in vivo has not been explored. Here we show that the axon guidance cue UNC-6/netrin and its receptor UNC-5 act throughout development to exclude synaptic vesicle and active zone proteins from the dendrite of the Caenorhabditis elegans motor neuron DA9, which is proximal to a source of UNC-6/netrin. In unc-6/netrin and unc-5 loss-of-function mutants, presynaptic components mislocalize to the DA9 dendrite. In addition, ectopically expressed UNC-6/netrin, acting through UNC-5, is sufficient to exclude endogenous synapses from adjacent subcellular domains within the DA9 axon. Furthermore, this anti-synaptogenic activity is interchangeable with that of LIN-44/Wnt despite being transduced through different receptors, suggesting that extracellular cues such as netrin and Wnts not only guide axon navigation but also regulate the polarized accumulation of presynaptic components through local exclusion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
02 October 2008
The AOP version of this paper contained inaccuracies in Figures 1 and 2. This was corrected for print on 2 October 2008.
References
Horton, A. C. & Ehlers, M. D. Neuronal polarity and trafficking. Neuron 40, 277–295 (2003)
White, J. G., Southgate, E., Thomson, J. N. & Brenner, S. The structure of the ventral nerve cord of Caenorhabditis elegans . Phil. Trans. R. Soc. Lond. B 275, 327–348 (1976)
Klassen, M. P. & Shen, K. Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans . Cell 130, 704–716 (2007)
Wadsworth, W. G., Bhatt, H. & Hedgecock, E. M. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans . Neuron 16, 35–46 (1996)
Mitchell, K. J. et al. Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron 17, 203–215 (1996)
Serafini, T. et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 87, 1001–1014 (1996)
Leung-Hagesteijn, C. et al. UNC-5, a transmembrane protein with immunoglobulin and thrombospondin type 1 domains, guides cell and pioneer axon migrations in C. elegans . Cell 71, 289–299 (1992)
Chan, S. S. et al. UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell 87, 187–195 (1996)
Keino-Masu, K. et al. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 87, 175–185 (1996)
Kolodziej, P. A. et al. frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell 87, 197–204 (1996)
Su, M. et al. Regulation of the UNC-5 netrin receptor initiates the first reorientation of migrating distal tip cells in Caenorhabditis elegans . Development 127, 585–594 (2000)
Fox, R. M. et al. A gene expression fingerprint of C. elegans embryonic motor neurons. BMC Genomics 6, 42 (2005)
Sym, M., Robinson, N. & Kenyon, C. MIG-13 positions migrating cells along the anteroposterior body axis of C. elegans . Cell 98, 25–36 (1999)
Jin, Y., Jorgensen, E., Hartwieg, E. & Horvitz, H. R. The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J. Neurosci. 19, 539–548 (1999)
Colavita, A., Krishna, S., Zheng, H., Padgett, R. W. & Culotti, J. G. Pioneer axon guidance by UNC-129, a C. elegans TGF-beta. Science (New York, N. Y 281, 706–709 (1998)
Sieburth, D. et al. Systematic analysis of genes required for synapse structure and function. Nature 436, 510–517 (2005)
Paganoni, S. & Ferreira, A. Expression and subcellular localization of Ror tyrosine kinase receptors are developmentally regulated in cultured hippocampal neurons. J. Neurosci. Res. 73, 429–440 (2003)
Phelan, P. Innexins: members of an evolutionarily conserved family of gap-junction proteins. Biochim. Biophys. Acta 1711, 225–245 (2005)
Whangbo, J. & Kenyon, C. A Wnt signaling system that specifies two patterns of cell migration in C. elegans . Mol. Cell 4, 851–858 (1999)
Colon-Ramos, D. A., Margeta, M. A. & Shen, K. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans . Science 318, 103–106 (2007)
Adler, C. E., Fetter, R. D. & Bargmann, C. I. UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nature Neurosci. 9, 511–518 (2006)
Manitt, C., Wang, D., Kennedy, T. E. & Howland, D. R. Positioned to inhibit: netrin-1 and netrin receptor expression after spinal cord injury. J. Neurosci. Res. 84, 1808–1820 (2006)
McAllister, A. K. Dynamic aspects of CNS synapse formation. Annu. Rev. Neurosci. 30, 425–450 (2007)
Hall, A. C., Lucas, F. R. & Salinas, P. C. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell 100, 525–535 (2000)
Packard, M. et al. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell 111, 319–330 (2002)
Umemori, H., Linhoff, M. W., Ornitz, D. M. & Sanes, J. R. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell 118, 257–270 (2004)
McCabe, B. D. et al. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron 39, 241–254 (2003)
Herman, M. A., Vassilieva, L. L., Horvitz, H. R., Shaw, J. E. & Herman, R. K. The C. elegans gene lin-44, which controls the polarity of certain asymmetric cell divisions, encodes a Wnt protein and acts cell nonautonomously. Cell 83, 101–110 (1995)
Mello, C. & Fire, A. DNA transformation. Methods Cell Biol. 48, 451–482 (1995)
Acknowledgements
This work was supported by the W. M. Keck Foundation, the McKnight Endowment Fund, the Searle Scholar Award and the Howard Hughes Medical Institute. We thank the International Caenorhabditis Genetic Center and M. Chalfie for strains. We further thank M. Chalfie for sharing unpublished results on the silencing system. We also thank C. Gao and Y. Fu for technical assistance, and T. Clandinin, C. Bargmann, S. McConnell, and members of the Shen laboratory for comments on the manuscript.
Author Contributions V.Y.P., M.P.K. and K.S. designed the experiments; V.Y.P. performed the experiments and data analysis; V.Y.P., M.P.K. and K.S. wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-12. (PDF 3682 kb)
Rights and permissions
About this article
Cite this article
Poon, V., Klassen, M. & Shen, K. UNC-6/netrin and its receptor UNC-5 locally exclude presynaptic components from dendrites. Nature 455, 669–673 (2008). https://doi.org/10.1038/nature07291
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature07291
This article is cited by
-
Specificity of synapse formation in Aplysia: paracrine and autocrine signaling regulates bidirectional molecular interactions between sensory and non-target motor neurons
Scientific Reports (2020)
-
Drosophila Netrin-B controls mushroom body axon extension and regulates courtship-associated learning and memory of a Drosophila fragile X syndrome model
Molecular Brain (2019)
-
Intrinsic and extrinsic mechanisms of synapse formation and specificity in C. elegans
Cellular and Molecular Life Sciences (2019)
-
The PHR proteins: intracellular signaling hubs in neuronal development and axon degeneration
Neural Development (2016)
-
Deep phenotyping unveils hidden traits and genetic relations in subtle mutants
Nature Communications (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.