Abstract
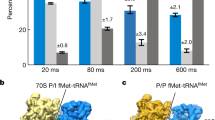
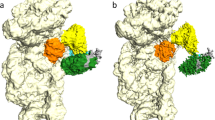
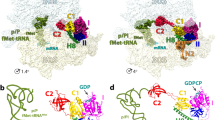
Translation initiation, the rate-limiting step of the universal process of protein synthesis, proceeds through sequential, tightly regulated steps. In bacteria, the correct messenger RNA start site and the reading frame are selected when, with the help of initiation factors IF1, IF2 and IF3, the initiation codon is decoded in the peptidyl site of the 30S ribosomal subunit by the fMet-tRNAfMet anticodon. This yields a 30S initiation complex (30SIC) that is an intermediate in the formation of the 70S initiation complex (70SIC) that occurs on joining of the 50S ribosomal subunit to the 30SIC and release of the initiation factors1,2,3. The localization of IF2 in the 30SIC has proved to be difficult so far using biochemical approaches, but could now be addressed using cryo-electron microscopy and advanced particle separation techniques on the basis of three-dimensional statistical analysis. Here we report the direct visualization of a 30SIC containing mRNA, fMet-tRNAfMet and initiation factors IF1 and GTP-bound IF2. We demonstrate that the fMet-tRNAfMet is held in a characteristic and precise position and conformation by two interactions that contribute to the formation of a stable complex: one involves the transfer RNA decoding stem which is buried in the 30S peptidyl site, and the other occurs between the carboxy-terminal domain of IF2 and the tRNA acceptor end. The structure provides insights into the mechanism of 70SIC assembly and rationalizes the rapid activation of GTP hydrolysis triggered on 30SIC–50S joining2,3 by showing that the GTP-binding domain of IF2 would directly face the GTPase-activated centre of the 50S subunit.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gualerzi, C. O. et al. Initiation factors in the early events of mRNA translation in bacteria. Cold Spring Harb. Symp. Quant. Biol. 66, 363–376 (2001)
Tomšic, J. et al. Late events of translation initiation in bacteria: a kinetic analysis. EMBO J. 19, 2127–2136 (2000)
Grigoriadou, C., Marzi, S., Kirillov, S., Gualerzi, C. O. & Cooperman, B. S. A quantitative kinetic scheme for 70 S translation initiation complex formation. J. Mol. Biol. 373, 562–572 (2007)
Caserta, E. et al. Translation initiation factor IF2 interacts with the 30 S ribosomal subunits via two separate binding sites. J. Mol. Biol. 362, 787–799 (2006)
Simonetti, A. et al. Nature Protocols 10.1038/nprot.2008.130 (2008)
Klaholz, B. P., Myasnikov, A. G. & van Heel, M. Visualization of release factor 3 on the ribosome during termination of protein synthesis. Nature 427, 862–865 (2004)
Wimberly, B. T. et al. Structure of the 30S ribosomal subunit. Nature 407, 327–339 (2000)
Schluenzen, F. et al. Structure of functionally activated small ribosomal subunit at 3.3 angstroms resolution. Cell 102, 615–623 (2000)
Korostelev, A., Trakhanov, S., Laurberg, M. & Noller, H. F. Crystal structure of a 70S ribosome-tRNA complex reveals functional interactions and rearrangements. Cell 126, 1065–1077 (2006)
Yusupova, G., Jenner, L., Rees, B., Moras, D. & Yusupov, M. Structural basis for messenger RNA movement on the ribosome. Nature 444, 391–394 (2006)
Carter, A. P. et al. Crystal structure of an initiation factor bound to the 30S ribosomal subunit. Science 291, 498–501 (2001)
Roll-Mecak, A., Cao, C., Dever, T. E. & Burley, S. K. X-ray structures of the universal translation initiation factor IF2/eIF5B: conformational changes on GDP and GTP binding complex. Cell 103, 781–792 (2000)
Myasnikov, A. G. et al. Conformational transition of initiation factor 2 from the GTP- to GDP-bound state visualized on the ribosome. Nature Struct. Mol. Biol. 12, 1145–1149 (2005)
Selmer, M. et al. Structure of the 70S ribosome complexed with mRNA and tRNA. Science 313, 1935–1942 (2006)
Szkaradkiewicz, K., Zuleeg, S., Limmer, S. & Sprinzl, M. Interaction of fMet-tRNAfMet and fMet-AMP with the C-terminal domain of Thermus thermophilus translation initiation factor 2. Eur. J. Biochem. 267, 4290–4299 (2000)
Guenneugues, M. et al. Mapping the fMet-tRNA binding site of initiation factor IF2. EMBO J. 19, 5233–5249 (2000)
Valle, M. et al. Cryo-EM reveals an active role for aminoacyl-tRNA in the accommodation process. EMBO J. 21, 3557–3567 (2002)
Moazed, D. & Noller, H. F. Intermediate states in the movement of transfer RNA in the ribosome. Nature 342, 142–148 (1989)
Allen, G. S., Zavialov, A., Gursky, R., Ehrenberg, M. & Frank, J. The cryo-EM structure of a translation initiation complex from Escherichia coli . Cell 121, 703–712 (2005)
Gualerzi, C. O. & Pon, C. L. Initiation of mRNA translation in prokaryotes. Biochemistry 29, 5881–5889 (1990)
Antoun, A., Pavlov, M. Y., Tenson, T. & Ehrenberg, M. Ribosome formation from subunits studied by stopped-flow and Rayleigh light scattering. Biol. Proceed. Online 6, 35–54 (2004)
Ali, I. K., Lancaster, L., Feinberg, J., Joseph, S. & Noller, H. F. Deletion of a conserved, central ribosomal intersubunit RNA bridge. Mol. Cell 23, 865–874 (2006)
Milon, P., Konevega, A. L., Gualerzi, C. O. & Rodnina, M. V. Kinetic checkpoint at a late step in translation initiation. Mol. Cell 30, 712–720 (2008)
Giuliodori, A. M., Giangrossi, M., Brandi, A., Gualerzi, C. O. & Pon, C. L. Cold-stress-induced de novo expression of infC and role of IF3 in cold-shock translational bias. RNA 13, 1355–1365 (2007)
Klaholz, B. P. et al. Structure of the Escherichia coli ribosomal termination complex with release factor 2. Nature 421, 90–94 (2003)
Marzi, S. et al. Structured mRNAs regulate translation initiation by binding to the platform of the ribosome. Cell 130, 1019–1031 (2007)
Efron, B. Nonparametric estimates of standard error: The jackknife, the bootstrap and other methods. Biometrika 68, 589–599 (1981)
Haynor, D. R. & Woods, S. D. Resampling estimates of precision in emission tomography. IEEE Trans. Med. Imaging 8, 337–343 (1989)
Penczek, P. A., Yang, C., Frank, J. & Spahn, C. M. Estimation of variance in single-particle reconstruction using the bootstrap technique. J. Struct. Biol. 154, 168–183 (2006)
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003)
van Heel, M. & Schatz, M. Fourier shell correlation threshold criteria. J. Struct. Biol. 151, 250–262 (2005)
Thompson, J. & Dahlberg, A. E. Testing the conservation of the translational machinery over evolution in diverse environments: assaying Thermus thermophilus ribosomes and initiation factors in a coupled transcription-translation system from Escherichia coli . Nucleic Acids Res. 32, 5954–5961 (2004)
Rodnina, M. V., Semenkov, Y. P. & Wintermeyer, W. Purification of fMettRNA(fMet) by fast protein liquid chromatography. Anal. Biochem. 219, 380–381 (1994)
Fechter, P. et al. Ribosomal initiation complexes probed by toeprinting and effect of trans-acting translational regulators in bacteria. Methods Mol. Biol. (in the press)
Yusupova, G. Z., Yusupov, M. M., Cate, J. H. & Noller, H. F. The path of the messenger RNA throught the ribosome. Cell 106, 233–241 (2001)
van Heel, M., Harauz, G., Orlova, E. V., Schmidt, R. & Schatz, M. A new generation of the IMAGIC image processing system. J. Struct. Biol. 116, 17–24 (1996)
Orlov, I. M., Morgan, D. G. & Cheng, R. H. Efficient implementation of a filtered back-projection algorithm using a voxel-by-voxel approach. J. Struct. Biol. 154, 287–296 (2006)
Acknowledgements
We thank J. Thompson for providing IF2 strains, M. Argentini for mass spectroscopy analysis of IF1 and IF2, and P. Schultz, D. Moras, J.-C. Thierry, V. Mallouh, G. Yusupova and I. Orlov for their constant support and interest. This work was supported by grants from the Centre National pour la Recherche Scientifique (CNRS), the Ministère de la Recherche et de la Technologie, the European Molecular Biology Organization Young Investigator Programme, the Institut du Développement et des Ressources en Informatique Scientifique, and the European Commission as SPINE2-complexes (contract no LSHG-CT-2006-031220). A.S. is a PhD student in a co-tutorial between the Université Louis Pasteur (ULP) and the Università di Camerino, and was supported by SPINE2-complexes, by the Institut National de la Santé et de la Recherche Médicale (INSERM) and by the Fondation de la Recherche Médicale (FRM). S.M. was supported by postdoctoral fellowships from the ULP, the CNRS and from the FRM, and A.G.M. was a recipient of postdoctoral fellowships from the CNRS and the FRM. The electron microscope facility is supported by the Alsace Region, the INSERM, the CNRS and the Association pour la Recherche sur le Cancer.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures S1-S2 with legends, and Supplementary References. (PDF 404 kb)
Rights and permissions
About this article
Cite this article
Simonetti, A., Marzi, S., Myasnikov, A. et al. Structure of the 30S translation initiation complex. Nature 455, 416–420 (2008). https://doi.org/10.1038/nature07192
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07192
This article is cited by
-
Thousands of human non-AUG extended proteoforms lack evidence of evolutionary selection among mammals
Nature Communications (2022)
-
Structural basis for ribosome recycling by RRF and tRNA
Nature Structural & Molecular Biology (2020)
-
A possible universal role for mRNA secondary structure in bacterial translation revealed using a synthetic operon
Nature Communications (2020)
-
eIF2A, an initiator tRNA carrier refractory to eIF2α kinases, functions synergistically with eIF5B
Cellular and Molecular Life Sciences (2018)
-
A conformational switch in initiation factor 2 controls the fidelity of translation initiation in bacteria
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.