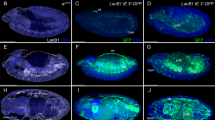
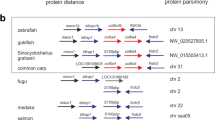
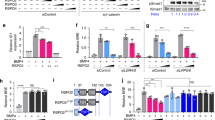
Abstract
Dorsal–ventral patterning in vertebrate and invertebrate embryos is mediated by a conserved system of secreted proteins that establishes a bone morphogenetic protein (BMP) gradient. Although the Drosophila embryonic Decapentaplegic (Dpp) gradient has served as a model to understand how morphogen gradients are established, no role for the extracellular matrix has been previously described. Here we show that type IV collagen extracellular matrix proteins bind Dpp and regulate its signalling in both the Drosophila embryo and ovary. We provide evidence that the interaction between Dpp and type IV collagen augments Dpp signalling in the embryo by promoting gradient formation, yet it restricts the signalling range in the ovary through sequestration of the Dpp ligand. Together, these results identify a critical function of type IV collagens in modulating Dpp in the extracellular space during Drosophila development. On the basis of our findings that human type IV collagen binds BMP4, we predict that this role of type IV collagens will be conserved.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hogan, B. L. Bone morphogenetic proteins in development. Curr. Opin. Genet. Dev. 6, 432–438 (1996)
Kishigami, S. & Mishina, Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 16, 265–278 (2005)
Chen, D., Zhao, M. & Mundy, G. R. Bone morphogenetic proteins. Growth Factors 22, 233–241 (2004)
Bobik, A. Transforming growth factor-βs and vascular disorders. Arterioscler. Thromb. Vasc. Biol. 26, 1712–1720 (2006)
Harradine, K. A. & Akhurst, R. J. Mutations of TGFβ signaling molecules in human disease. Ann. Med. 38, 403–414 (2006)
Reddi, A. H. BMPs: from bone morphogenetic proteins to body morphogenetic proteins. Cytokine Growth Factor Rev. 16, 249–250 (2005)
Bessa, P. C., Casal, M. & Reis, R. L. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery). J. Tissue Eng. Regen. Med. 2, 81–96 (2008)
Podos, S. D. & Ferguson, E. L. Morphogen gradients: new insights from DPP. Trends Genet. 15, 396–402 (1999)
Ashe, H. L. BMP signalling: synergy and feedback create a step gradient. Curr. Biol. 15, R375–R377 (2005)
Kirilly, D. & Xie, T. The Drosophila ovary: an active stem cell community. Cell Res. 17, 15–25 (2007)
Eldar, A. et al. Robustness of the BMP morphogen gradient in Drosophila embryonic patterning. Nature 419, 304–308 (2002)
Shimmi, O., Umulis, D., Othmer, H. & O’Connor, M. B. Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell 120, 873–886 (2005)
Wang, Y. C. & Ferguson, E. L. Spatial bistability of Dpp-receptor interactions during Drosophila dorsal–ventral patterning. Nature 434, 229–234 (2005)
Khoshnoodi, J., Cartailler, J. P., Alvares, K., Veis, A. & Hudson, B. G. Molecular recognition in the assembly of collagens: terminal noncollagenous domains are key recognition modules in the formation of triple helical protomers. J. Biol. Chem. 281, 38117–38121 (2006)
Yasothornsrikul, S., Davis, W. J., Cramer, G., Kimbrell, D. A. & Dearolf, C. R. viking: identification and characterization of a second type IV collagen in Drosophila . Gene 198, 17–25 (1997)
Blumberg, B., MacKrell, A. J. & Fessler, J. H. Drosophila basement membrane procollagen α1(IV). Complete cDNA sequence, genomic structure, and general implications for supramolecular assemblies. J. Biol. Chem. 263, 18328–18337 (1988)
Ross, J. J. et al. Twisted gastrulation is a conserved extracellular BMP antagonist. Nature 410, 479–483 (2001)
Knibiehler, B., Mirre, C. & Le Parco, Y. Collagen type IV of Drosophila is stockpiled in the growing oocyte and differentially located during early stages of embryogenesis. Cell Differ. Dev. 30, 147–157 (1990)
Medioni, C. & Noselli, S. Dynamics of the basement membrane in invasive epithelial clusters in Drosophila . Development 132, 3069–3077 (2005)
Morin, X., Daneman, R., Zavortink, M. & Chia, W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila . Proc. Natl Acad. Sci. USA 98, 15050–15055 (2001)
Bhat, M. A. et al. Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell 96, 833–845 (1999)
Lunstrum, G. P. et al. Drosophila basement membrane procollagen IV. J. Biol. Chem. 263, 18318–18327 (1988)
Ashe, H. L., Mannervik, M. & Levine, M. Dpp signaling thresholds in the dorsal ectoderm of the Drosophila embryo. Development 127, 3305–3312 (2000)
Ashe, H. L. & Levine, M. Local inhibition and long-range enhancement of Dpp signal transduction by Sog. Nature 398, 427–431 (1999)
Xie, T. & Spradling, A. C. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell 94, 251–260 (1998)
Larrain, J. et al. BMP-binding modules in chordin: a model for signalling regulation in the extracellular space. Development 127, 821–830 (2000)
Maduzia, L. L. & Padgett, R. W. Drosophila MAD, a member of the Smad family, translocates to the nucleus upon stimulation of the dpp pathway. Biochem. Biophys. Res. Commun. 238, 595–598 (1997)
Lee, H. S., Simon, J. A. & Lis, J. T. Structure and expression of ubiquitin genes of Drosophila melanogaster . Mol. Cell. Biol. 8, 4727–4735 (1988)
Schock, F. & Perrimon, N. Retraction of the Drosophila germ band requires cell–matrix interaction. Genes Dev. 17, 597–602 (2003)
Reed, B. H., Wilk, R., Schock, F. & Lipshitz, H. D. Integrin-dependent apposition of Drosophila extraembryonic membranes promotes morphogenesis and prevents anoikis. Curr. Biol. 14, 372–380 (2004)
Belenkaya, T. Y. et al. Drosophila Dpp morphogen movement is independent of dynamin-mediated endocytosis but regulated by the glypican members of heparan sulfate proteoglycans. Cell 119, 231–244 (2004)
Abe, H. et al. Type IV collagen is transcriptionally regulated by Smad1 under advanced glycation end product (AGE) stimulation. J. Biol. Chem. 279, 14201–14206 (2004)
Paralkar, V. M., Weeks, B. S., Yu, Y. M., Kleinman, H. K. & Reddi, A. H. Recombinant human bone morphogenetic protein 2B stimulates PC12 cell differentiation: potentiation and binding to type IV collagen. J. Cell Biol. 119, 1721–1728 (1992)
Yu, K. et al. Processing of the Drosophila Sog protein creates a novel BMP inhibitory activity. Development 127, 2143–2154 (2000)
Srinivasan, S., Rashka, K. E. & Bier, E. Creation of a Sog morphogen gradient in the Drosophila embryo. Dev. Cell 2, 91–101 (2002)
Persson, U. et al. The L45 loop in type I receptors for TGF-β family members is a critical determinant in specifying Smad isoform activation. FEBS Lett. 434, 83–87 (1998)
McKearin, D. & Ohlstein, B. A role for the Drosophila bag-of-marbles protein in the differentiation of cystoblasts from germline stem cells. Development 121, 2937–2947 (1995)
Acknowledgements
We thank M. O’Connor for plasmids, flies and advice on Dpp–HA staining; W. Chia, R. Ray and the Bloomington stock centre for fly stocks; E. Bier, P. ten Dijke, H. Bellen, L. Fessler and D. McKearin for antibodies; T. Jowitt for help with SPR; S. Lunj, M. Ronshaugen and W. Song for help; M. Ashe and W. Miles for reading the manuscript; and M. Levine whose laboratory this work was initiated in. This work was funded by a BBSRC project grant to H.L.A. and a BBSRC studentship to R.E.H.
Author Contributions H.L.A. designed and obtained funding for the experiments. X.W. generated the in vitro and embryonic data. R.E.H. performed the ovary experiments. L.J.B. provided technical assistance with cloning, fly counts and transgenics. X.W., R.E.H. and H.L.A. analysed and interpreted the data, and H.L.A. wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-10 with Legends and additional references The data include further characterisation of the following: collagen IV-Dpp/BMP interaction; collagen IV expression and distribution in the embryo; and phenotype of type IV collagen mutant embryos. (PDF 2500 kb)
Rights and permissions
About this article
Cite this article
Wang, X., Harris, R., Bayston, L. et al. Type IV collagens regulate BMP signalling in Drosophila. Nature 455, 72–77 (2008). https://doi.org/10.1038/nature07214
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07214
This article is cited by
-
Screening of hub inflammatory bowel disease biomarkers and identification of immune-related functions based on basement membrane genes
European Journal of Medical Research (2023)
-
Single-molecule tracking of Nodal and Lefty in live zebrafish embryos supports hindered diffusion model
Nature Communications (2022)
-
Mechanisms underlying pre- and postnatal development of the vomeronasal organ
Cellular and Molecular Life Sciences (2021)
-
Drosophila ML-DmD17-c3 cells respond robustly to Dpp and exhibit complex transcriptional feedback on BMP signaling components
BMC Developmental Biology (2019)
-
The calcium channel subunit α2δ-3 organizes synapses via an activity-dependent and autocrine BMP signaling pathway
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.