Abstract
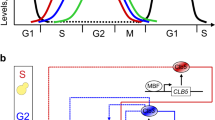
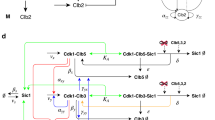
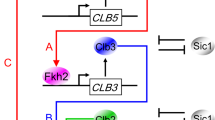
A significant fraction of the Saccharomyces cerevisiae genome is transcribed periodically during the cell division cycle1,2, indicating that properly timed gene expression is important for regulating cell-cycle events. Genomic analyses of the localization and expression dynamics of transcription factors suggest that a network of sequentially expressed transcription factors could control the temporal programme of transcription during the cell cycle3. However, directed studies interrogating small numbers of genes indicate that their periodic transcription is governed by the activity of cyclin-dependent kinases (CDKs)4. To determine the extent to which the global cell-cycle transcription programme is controlled by cyclin–CDK complexes, we examined genome-wide transcription dynamics in budding yeast mutant cells that do not express S-phase and mitotic cyclins. Here we show that a significant fraction of periodic genes are aberrantly expressed in the cyclin mutant. Although cells lacking cyclins are blocked at the G1/S border, nearly 70% of periodic genes continued to be expressed periodically and on schedule. Our findings reveal that although CDKs have a function in the regulation of cell-cycle transcription, they are not solely responsible for establishing the global periodic transcription programme. We propose that periodic transcription is an emergent property of a transcription factor network that can function as a cell-cycle oscillator independently of, and in tandem with, the CDK oscillator.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Primary accessions
Gene Expression Omnibus
Data deposits
The microarray data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO series accession number GSE8799.
References
Pramila, T., Wu, W., Miles, S., Noble, W. S. & Breeden, L. L. The Forkhead transcription factor Hcm1 regulates chromosome segregation genes and fills the S-phase gap in the transcriptional circuitry of the cell cycle. Genes Dev. 20, 2266–2278 (2006)
Spellman, P. T. et al. Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol. Biol. Cell 9, 3273–3297 (1998)
Simon, I. et al. Serial regulation of transcriptional regulators in the yeast cell cycle. Cell 106, 697–708 (2001)
Wittenberg, C. & Reed, S. I. Cell cycle-dependent transcription in yeast: Promoters, transcription factors, and transcriptomes. Oncogene 24, 2746–2755 (2005)
Murray, A. W. Recycling the cell cycle: Cyclins revisited. Cell 116, 221–234 (2004)
Zwicker, J. & Muller, R. Cell cycle-regulated transcription in mammalian cells. Prog. Cell Cycle Res. 1, 91–99 (1995)
Cho, R. J. et al. A genome-wide transcriptional analysis of the mitotic cell cycle. Mol. Cell 2, 65–73 (1998)
Ren, B. et al. Genome-wide location and function of DNA binding proteins. Science 290, 2306–2309 (2000)
Lee, T. I. et al. Transcriptional regulatory networks in Saccharomyces cerevisiae . Science 298, 799–804 (2002)
Haase, S. B. & Reed, S. I. Evidence that a free-running oscillator drives G1 events in the budding yeast cell cycle. Nature 401, 394–397 (1999)
Haase, S. B., Winey, M. & Reed, S. I. Multi-step control of spindle pole body duplication by cyclin-dependent kinase. Nature Cell Biol. 3, 38–42 (2001)
Lew, D. J. & Reed, S. I. Morphogenesis in the yeast cell cycle: Regulation by Cdc28 and cyclins. J. Cell Biol. 120, 1305–1320 (1993)
de Lichtenberg, U. et al. Comparison of computational methods for the identification of cell cycle-regulated genes. Bioinformatics 21, 1164–1171 (2005)
Amon, A., Tyers, M., Futcher, B. & Nasmyth, K. Mechanisms that help the yeast cell cycle clock tick: G2 cyclins transcriptionally activate G2 cyclins and repress G1 cyclins. Cell 74, 993–1007 (1993)
Koch, C., Schleiffer, A., Ammerer, G. & Nasmyth, K. Switching transcription on and off during the yeast cell cycle: Cln/Cdc28 kinases activate bound transcription factor SBF (Swi4/Swi6) at start, whereas Clb/Cdc28 kinases displace it from the promoter in G2. Genes Dev. 10, 129–141 (1996)
Toyn, J. H., Johnson, A. L., Donovan, J. D., Toone, W. M. & Johnston, L. H. The Swi5 transcription factor of Saccharomyces cerevisiae has a role in exit from mitosis through induction of the CDK-inhibitor Sic1 in telophase. Genetics 145, 85–96 (1997)
Knapp, D., Bhoite, L., Stillman, D. J. & Nasmyth, K. The transcription factor Swi5 regulates expression of the cyclin kinase inhibitor p40SIC1. Mol. Cell. Biol. 16, 5701–5707 (1996)
Moll, T., Tebb, G., Surana, U., Robitsch, H. & Nasmyth, K. The role of phosphorylation and the CDC28 protein kinase in cell cycle-regulated nuclear import of the S. cerevisiae transcription factor, SWI5. Cell 66, 743–758 (1991)
O’Conallain, C., Doolin, M. T., Taggart, C., Thornton, F. & Butler, G. Regulated nuclear localisation of the yeast transcription factor Ace2p controls expression of chitinase (CTS1) in Saccharomyces cerevisiae . Mol. Gen. Genet. 262, 275–282 (1999)
Zhu, G. et al. Two yeast forkhead genes regulate the cell cycle and pseudohyphal growth. Nature 406, 90–94 (2000)
Kumar, R. et al. Forkhead transcription factors, Fkh1p and Fkh2p, collaborate with Mcm1p to control transcription required for M-phase. Curr. Biol. 10, 896–906 (2000)
Frey, B. J. & Dueck, D. Clustering by passing messages between data points. Science 315, 972–976 (2007)
Harbison, C. T. et al. Transcriptional regulatory code of a eukaryotic genome. Nature 431, 99–104 (2004)
Costanzo, M. et al. CDK activity antagonizes Whi5, an inhibitor of G1/S transcription in yeast. Cell 117, 899–913 (2004)
de Bruin, R. A. et al. Constraining G1-specific transcription to late G1 phase: the MBF-associated corepressor Nrm1 acts via negative feedback. Mol. Cell 23, 483–496 (2006)
Ho, Y., Costanzo, M., Moore, L., Kobayashi, R. & Andrews, B. J. Regulation of transcription at the Saccharomyces cerevisiae start transition by Stb1, a Swi6-binding protein. Mol. Cell. Biol. 19, 5267–5278 (1999)
Pic-Taylor, A., Darieva, Z., Morgan, B. A. & Sharrocks, A. D. Regulation of cell cycle-specific gene expression through cyclin-dependent kinase-mediated phosphorylation of the forkhead transcription factor Fkh2p. Mol. Cell. Biol. 24, 10036–10046 (2004)
Sidorova, J. M., Mikesell, G. E. & Breeden, L. L. Cell cycle-regulated phosphorylation of Swi6 controls its nuclear localization. Mol. Biol. Cell 6, 1641–1658 (1995)
Ubersax, J. A. et al. Targets of the cyclin-dependent kinase Cdk1. Nature 425, 859–864 (2003)
Teixeira, M. C. et al. The YEASTRACT database: A tool for the analysis of transcription regulatory associations in Saccharomyces cerevisiae . Nucleic Acids Res. 34, D446–D451 (2006)
Li, C. & Wong, W. H. Model-based analysis of oligonucleotide arrays: model validation, design issues and standard error application. Genome Biol. 2 research0032.1–0032.11 doi: 10.1186/gb-2001-2-8-research0032 (2001)
Orlando, D. A. et al. A probabilistic model for cell cycle distributions in synchrony experiments. Cell Cycle 6, 478–488 (2007)
Acknowledgements
We thank D. Lew and L. Simmons Kovacs for discussions and critical reading of the manuscript, and P. Benfey for helpful discussions and support. Financial support was provided by the American Cancer Society (to S.B.H.), the Alfred P. Sloan Foundation (to A.J.H.), the National Science Foundation (to A.J.H. and J.E.S.S.) and the National Institutes of Health (to S.B.H., A.J.H. and J.E.S.S.).
Author Contributions D.A.O., C.Y.L. and S.B.H. designed and performed the experiments. J.Y.W. provided technical expertise. D.A.O., C.Y.L., A.B., E.S.I. and A.J.H. performed the computational analyses, with contributions from J.E.S.S. and S.B.H. to the boolean model. D.A.O. and S.B.H. prepared the manuscript with contributions from C.Y.L., A.B., E.S.I. and A.J.H.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-4, 6-15, 17, and 19 with Legends. It also contains Supplementary Methods with more complete details on the methods/analyses presented in the main manuscript. Supplementary Tables 3-9 with Legends, as well as Legends for Supplementary Tables 1 and 2 are included. (PDF 3721 kb)
Supplementary Figure 5
The file contains Supplementary Figure 5 with Legend. Each page (30 pages) displays the transcript profile of every gene from a wild-type cluster and its component cyclin mutant sub-clusters from Figure 3 as line-graphs with cluster centroids and within cluster Pearson correlations provided. (PDF 279 kb)
Supplementary Figure 16
The file contains Supplementary Figure 16 with Legend. Each page (11 pages) displays the transcript profile of every gene from a wild-type cluster and its component cyclin mutant sub-clusters from Supplementary Figure 15 as line-graphs with cluster centroids and within cluster Pearson correlations provided. (PDF 191 kb)
Supplementary Figure 18
The file contains Supplementary Figure 18 with Legend. Each page (9 pages) displays the transcript profile of every gene from a wild-type cluster and its component cyclin mutant sub-clusters from Supplementary Figure 17 as line-graphs with cluster centroids and within cluster Pearson correlations provided. (PDF 179 kb)
Supplementary Table 1
The file contains Supplementary Table 1. (XLS 302 kb)
Supplementary Table 2
The file contains Supplementary Table 2. (XLS 125 kb)
Rights and permissions
About this article
Cite this article
Orlando, D., Lin, C., Bernard, A. et al. Global control of cell-cycle transcription by coupled CDK and network oscillators. Nature 453, 944–947 (2008). https://doi.org/10.1038/nature06955
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature06955
This article is cited by
-
Conservation of dynamic characteristics of transcriptional regulatory elements in periodic biological processes
BMC Bioinformatics (2022)
-
Periodic synchronization of isolated network elements facilitates simulating and inferring gene regulatory networks including stochastic molecular kinetics
BMC Bioinformatics (2022)
-
Peroxiredoxins couple metabolism and cell division in an ultradian cycle
Nature Chemical Biology (2021)
-
Cyclin/Forkhead-mediated coordination of cyclin waves: an autonomous oscillator rationalizing the quantitative model of Cdk control for budding yeast
npj Systems Biology and Applications (2021)
-
Using extremal events to characterize noisy time series
Journal of Mathematical Biology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.