Abstract
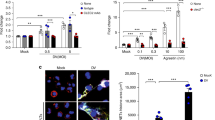
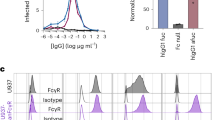
Dengue haemorrhagic fever and dengue shock syndrome, the most severe responses to dengue virus (DV) infection, are characterized by plasma leakage (due to increased vascular permeability) and low platelet counts1,2. CLEC5A (C-type lectin domain family 5, member A; also known as myeloid DAP12-associating lectin (MDL-1))3 contains a C-type lectin-like fold similar to the natural-killer T-cell C-type lectin domains and associates with a 12-kDa DNAX-activating protein (DAP12)4 on myeloid cells. Here we show that CLEC5A interacts with the dengue virion directly and thereby brings about DAP12 phosphorylation. The CLEC5A–DV interaction does not result in viral entry but stimulates the release of proinflammatory cytokines. Blockade of CLEC5A–DV interaction suppresses the secretion of proinflammatory cytokines without affecting the release of interferon-α, supporting the notion that CLEC5A acts as a signalling receptor for proinflammatory cytokine release. Moreover, anti-CLEC5A monoclonal antibodies inhibit DV-induced plasma leakage, as well as subcutaneous and vital-organ haemorrhaging, and reduce the mortality of DV infection by about 50% in STAT1-deficient mice. Our observation that blockade of CLEC5A-mediated signalling attenuates the production of proinflammatory cytokines by macrophages infected with DV (either alone or complexed with an enhancing antibody) offers a promising strategy for alleviating tissue damage and increasing the survival of patients suffering from dengue haemorrhagic fever and dengue shock syndrome, and possibly even other virus-induced inflammatory diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wilder-Smith, A. & Schwartz, E. Dengue in travelers. N. Engl. J. Med. 353, 924–932 (2005)
Mackenzie, J. S., Gubler, D. J. & Petersen, L. R. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nature Med. 10, S98–S109 (2004)
Bakker, A. B., Baker, E., Sutherland, G. R., Phillips, J. H. & Lanier, L. L. Myeloid DAP12-associating lectin (MDL)-1 is a cell surface receptor involved in the activation of myeloid cells. Proc. Natl Acad. Sci. USA 96, 9792–9796 (1999)
Lanier, L. L., Corliss, B. C., Wu, J., Leong, C. & Phillips, J. H. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature 391, 703–707 (1998)
Pang, T., Cardosa, M. J. & Guzman, M. G. Of cascades and perfect storms: the immunopathogenesis of dengue haemorrhagic fever–dengue shock syndrome (DHF/DSS). Immunol. Cell Biol. 85, 43–45 (2007)
Halstead, S. B. & O’Rourke, E. J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J. Exp. Med. 146, 201–217 (1977)
Palucka, A. K. Dengue virus and dendritic cells. Nature Med. 6, 748–749 (2000)
Wu, S. J. et al. Human skin Langerhans cells are targets of dengue virus infection. Nature Med. 6, 816–820 (2000)
Palmer, D. R. et al. Differential effects of dengue virus on infected and bystander dendritic cells. J. Virol. 79, 2432–2439 (2005)
Chen, Y. C. & Wang, S. Y. Activation of terminally differentiated human monocytes/macrophages by dengue virus: productive infection, hierarchical production of innate cytokines and chemokines, and the synergistic effect of lipopolysaccharide. J. Virol. 76, 9877–9887 (2002)
Cook, D. N., Pisetsky, D. S. & Schwartz, D. A. Toll-like receptors in the pathogenesis of human disease. Nature Immunol. 5, 975–979 (2004)
Klesney-Tait, J., Turnbull, I. R. & Colonna, M. The TREM receptor family and signal integration. Nature Immunol. 7, 1266–1273 (2006)
Robinson, M. J., Sancho, D., Slack, E. C., LeibundGut-Landmann, S. & Reis e Sousa, C. Myeloid C-type lectins in innate immunity. Nature Immunol. 7, 1258–1265 (2006)
Pokidysheva, E. et al. Cryo-EM reconstruction of dengue virus in complex with the carbohydrate recognition domain of DC-SIGN. Cell 124, 485–493 (2006)
Brown, G. D. & Gordon, S. A new receptor for β-glucans. Nature 413, 36–37 (2001)
Modis, Y., Ogata, S., Clements, D. & Harrison, S. C. Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. J. Virol. 79, 1223–1231 (2005)
Mitchell, D. A., Fadden, A. J. & Drickamer, K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J. Biol. Chem. 276, 28939–28945 (2001)
Lozach, P. Y. et al. Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN)-mediated enhancement of dengue virus infection is independent of DC-SIGN internalization signals. J. Biol. Chem. 280, 23698–23708 (2005)
Tassaneetrithep, B. et al. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 197, 823–829 (2003)
Johnson, A. J., Guirakhoo, F. & Roehrig, J. T. The envelope glycoproteins of dengue 1 and dengue 2 viruses grown in mosquito cells differ in their utilization of potential glycosylation sites. Virology 203, 241–249 (1994)
Goncalvez, A. P., Engle, R. E., St Claire, M., Purcell, R. H. & Lai, C. J. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc. Natl Acad. Sci. USA 104, 9422–9427 (2007)
Huang, K. J. et al. The dual-specific binding of dengue virus and target cells for the antibody-dependent enhancement of dengue virus infection. J. Immunol. 176, 2825–2832 (2006)
Durbin, J. E., Hackenmiller, R., Simon, M. C. & Levy, D. E. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell 84, 443–450 (1996)
Shresta, S. et al. Critical roles for both STAT1-dependent and STAT1-independent pathways in the control of primary dengue virus infection in mice. J. Immunol. 175, 3946–3954 (2005)
Arrighi, J. F. et al. Lentivirus-mediated RNA interference of DC-SIGN expression inhibits human immunodeficiency virus transmission from dendritic cells to T cells. J. Virol. 78, 10848–10855 (2004)
Lin, Y. L. et al. Study of Dengue virus infection in SCID mice engrafted with human K562 cells. J. Virol. 72, 9729–9737 (1998)
Shresta, S., Sharar, K. L., Prigozhin, D. M., Beatty, P. R. & Harris, E. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J. Virol. 80, 10208–10217 (2006)
Chang, Y. C. et al. Modulation of macrophage differentiation and activation by decoy receptor 3. J. Leukoc. Biol. 75, 486–494 (2004)
Hsu, T. L. et al. Modulation of dendritic cell differentiation and maturation by decoy receptor 3. J. Immunol. 168, 4846–4853 (2002)
Oshiumi, H., Matsumoto, M., Funami, K., Akazawa, T. & Seya, T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-β induction. Nature Immunol. 4, 161–167 (2003)
Acknowledgements
We thank W.-C. Yeh, C. Milner and J. Paulson for critical comments; N.-J. Chen, C.-H. Lin, Y-L. Lee and J.-J. Liang for technical assistance. Resources and collaborative efforts were provided by the RNAi Consortium, Academia Sinica, Taiwan, and the Consortium for Functional Glycomics funded by the National Institute of General Medical Sciences (GM62116). This work was supported mainly by the National Research Program for Genomic Medicine, National Science Council, Taiwan (NSC-95-3112-B-010-0171 and NSC 96-3112-B-010-2), and in part by the National Yang-Ming University, Taiwan (96A-D-D132 from the Ministry of Education), Taipei Veterans General Hospital (V97S5-001), and Academia Sinica.
Author Contributions S.-T.C. designed, performed and analysed experiments, and wrote the paper. Y.-L.L. designed, analysed experiments and wrote the paper. M.-T.H., M.-F.W. and S.-C.C. performed experiments. H.-Y.L., C.-K.L. and T.-W.C. provided materials and reagents. C.-H.W. analysed experiments. S.-L.H. designed and analysed experiments, and wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
The file contains Supplementary Tables 1-3 and Supplementary Figures 1-8 with Legends. (PDF 2199 kb)
Rights and permissions
About this article
Cite this article
Chen, ST., Lin, YL., Huang, MT. et al. CLEC5A is critical for dengue-virus-induced lethal disease. Nature 453, 672–676 (2008). https://doi.org/10.1038/nature07013
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07013
This article is cited by
-
Extracellular vesicles as tools and targets in therapy for diseases
Signal Transduction and Targeted Therapy (2024)
-
CLEC5A mediates Zika virus-induced testicular damage
Journal of Biomedical Science (2023)
-
Identification and validation of ferroptosis-related genes in patients infected with dengue virus: implication in the pathogenesis of DENV
Virus Genes (2023)
-
CLEC5A and TLR2 are critical in SARS-CoV-2-induced NET formation and lung inflammation
Journal of Biomedical Science (2022)
-
C-type lectin domain-containing protein CLEC3A regulates proliferation, regeneration and maintenance of nucleus pulposus cells
Cellular and Molecular Life Sciences (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.