Abstract
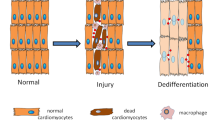
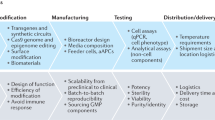
An improved understanding of stem-cell and regenerative biology, as well as a better control of stem-cell fate, is likely to produce treatments for many devastating diseases and injuries. Chemical approaches are starting to have an increasingly important role in this young field. Attention has focused on chemical approaches that allow the precise manipulation of cells in vitro to obtain homogeneous cell types for cell-based therapies. Another promising approach is the development of conventional chemical and biological therapeutics to stimulate endogenous cells to regenerate. Such therapeutics can act on target cells or their niches in vivo to promote cell survival, proliferation, differentiation, reprogramming and homing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Boyer, L. A. et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122, 947–956 (2005). This paper reports a genome-wide location analysis of the genes Oct4, Sox2 and Nanog , and proposes a model of core ES-cell regulatory circuitry for maintaining the pluripotent state of ES cells.
Lee, T. I. et al. Control of developmental regulator's by polycomb in human embryonic stem cells. Cell 125, 301–313 (2006).
Bernstein, B. E. et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326 (2006).
Mikkelsen, T. S. et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448, 553–560 (2007).
Scadden, D. T. The stem-cell niche as an entity of action. Nature 441, 1075–1079 (2006).
Chen, S. B. et al. Self-renewal of embryonic stem cells by a small molecule. Proc. Natl Acad. Sci. USA 103, 17266–17271 (2006). This paper describes the identification of a novel synthetic small molecule that can maintain long-term self-renewal of mouse ES cells in the absence of feeder cells, serum, LIF, BMPs and WNT proteins.
Ludwig, T. E. et al. Derivation of human embryonic stem cells in defined conditions. Nature Biotechnol. 24, 185–187 (2006).
Yao, S. et al. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc. Natl Acad. Sci. USA 103, 6907–6912 (2006).
D'Amour, K. A. et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nature Biotechnol. 24, 1392–1401 (2006). This paper elegantly describes a directed, stepwise differentiation of human ES cells into functional pancreatic hormone-expressing endocrine cells.
Chen, S. B., Zhang, Q. S., Wu, X., Schultz, P. G. & Ding, S. Dedifferentiation of lineage-committed cells by a small molecule. J. Am. Chem. Soc. 126, 410–411 (2004).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006). This paper was the first to demonstrate that mouse somatic cells can be reprogrammed to become iPS cells by viral transduction of four defined factors: OCT4, SOX2, KLF4 and Myc.
Ding, S. & Schultz, P. G. A role for chemistry in stem cell biology. Nature Biotechnol. 22, 833–840 (2004).
Keller, G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 19, 1129–1155 (2005).
Shamblott, M. J. et al. Derivation of pluripotent stem cells horn cultured human primordial germ cells. Proc. Natl Acad. Sci. USA 95, 13726–13731 (1998).
Kanatsu-Shinohara, M. et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell 119, 1001–1012 (2004).
Guan, K. et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature 440, 1199–1203 (2006).
Brons, I. G. M. et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448, 191–195 (2007).
Tesar, P. J. et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448, 196–199 (2007). References 17 and 18 report the derivation of pluripotent epiblast stem cells from post-implantation, epiblast-stage embryos of mice and rats.
Wu, H. et al. Integrative genomic and functional analyses reveal neuronal subtype differentiation bias in human embryonic stem cell lines. Proc. Natl Acad. Sci. USA 104, 13821–13826 (2007).
Ying, Q. L., Nichols, J., Chambers, I. & Smith, A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 115, 281–292 (2003).
Vallier, L., Reynolds, D. & Pederson, R. A. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev. Biol. 275, 403–421 (2004).
Xu, R. H. et al. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nature Methods 2, 185–190 (2005).
Beattie, G. M. et al. Activin A maintains pluripotency of human embryonic stem cells in the sbsence of feeder layers. Stem Cells 23, 489–495 (2005).
Lu, J., Hou, R., Booth, C. J., Yang, S.-H. & Snyder, M. Defined culture conditions of human embryonic stem cells. Proc. Natl Acad. Sci. USA 103, 5688–5693 (2006).
Wang, L. et al. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood 110, 4111–4119 (2007).
Watanabe, K. et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nature Biotechnol. 25, 681–686 (2007).
Conti, L. et al. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PloS Biol. 3, 1594–1606 (2005).
Qyang, Y. et al. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/β-catenin pathway. Cell Stem Cell 1, 165–179 (2007).
Moretti, A. et al. Multipotent embryonic Isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 127, 1151–1165 (2006).
Kawasaki, H. et al. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron 28, 31–40 (2000).
Perrier, A. L. et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc. Natl Acad. Sci. USA 101, 12543–12548 (2004).
Wichterle, H., Lieberam, I., Porter, J. A. & Jessell, T. M. Directed differentiation of embryonic stem cells into motor neurons. Cell 110, 385–397 (2002).
Ying, Q. L., Stavridis, M., Griffiths, D., Li, M. & Smith, A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nature Biotechnol. 21, 183–186 (2003).
Li, X. J. et al. Specification of motoneurons from human embryonic stem cells. Nature Biotechnol. 23, 215–221 (2005).
Kattman, S. J., Huber, T. L. & Keller, G. M. Multipotent Flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev. Cell 11, 723–732 (2006).
Laflamme, M. A. et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nature Biotechnol. 25, 1015–1024 (2007).
D'Amour, K. A. et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nature Biotechnol. 23, 1534–1541 (2005).
Warashina, M. et al. A synthetic small molecule that induces neuronal differentiation of adult hippocampal neural progenitor cells. Angew. Chemie Int. Edn Engl. 45, 591–593 (2006).
Diamandis, P. et al. Chemical genetics reveals a complex functional ground state of neural stem cells. Nature Chem. Biol. 3, 268–273 (2007).
Saxe, J. P. et al. A phenotypic small-molecule screen identifies an orphan ligand-receptor pair that regulates neural stem cell differentiation. Chem. Biol. 14, 1019–1030 (2007).
Hsieh, J., Nakashima, K., Kuwabara, T., Mejia, E. & Gage, F. H. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc. Natl Acad. Sci. USA 101, 16659–16664 (2004).
Santarelli, L. et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809 (2003).
Hochedlinger, K. & Jaenisch, R. Nuclear reprogramming and pluripotency. Nature 441, 1061–1067 (2006).
Eggan, E. et al. Mice cloned from olfactory sensory neurons. Nature 428, 44–49 (2004).
Ying, Q. L., Nichols, J., Evans, E. P. & Smith, A. G. Changing potency by spontaneous fusion. Nature 416, 545–548 (2002).
Do, J. T. & Scholer, H. R. Nuclei of embryonic stem cells reprogram somatic cells. Stem Cells 22, 941–949 (2004).
Cowan, C. A., Atienza, J., Melton, D. A. & Eggan, K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science 309, 1369–1373 (2005).
Cobaleda, C., Jochum, W. & Busslinger, M. Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. Nature 449, 473–477 (2007).
Cobaleda, C., Schebesta, A., Delogu, A. & Busslinger, M. Pax5: the guardian of B cell identity and function. Nature Immunol. 8, 463–470 (2007).
Kondo, T. & Raff, M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science 289, 1754–1757 (2000).
Shen, C. N., Slack, J. M. W. & Tosh, D. Molecular basis of transdifferentiation of pancreas to liver. Nature Cell Biol. 2, 879–887 (2000).
Egli, D., Rosains, J., Birkhoff, G. & Eggan, K. Developmental reprogramming after chromosome transfer into mitotic mouse zygotes. Nature 447, 679–685 (2007).
Byrne, J. A. et al. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature 450, 497–502 (2007).
Yamanaka, S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell 1, 39–49 (2007).
Okita, K., Ichisaka, T. & Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 448, 313–317 (2007).
Meissner, A., Wernig, M. & Jaenisch, R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nature Biotechnol. 25, 1177–1181 (2007).
Maherali, N. et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 1, 55–70 (2007).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Zhang, F., Pomerantz, J. H., Sen, G., Palermo, A. T., & Blau, H. M. Active tissue-specific DNA demethylation conferred by somatic cell nuclei in stable heterokaryons. Proc. Natl Acad. Sci. USA 104, 4395–400 (2007).
Horb, M. E., Shen, C. N., Tosh, D. & Slack, J. M. W. Experimental conversion of liver to pancreas. Curr. Biol. 13, 105–115 (2003).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Chen, S. B. et al. Reversine increases the plasticity of lineage-committed mammalian cells. Proc. Natl Acad. Sci. USA 104, 10482–10487 (2007).
North, T. E. et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 447, 1007–1011 (2007). This paper describes a chemical screen in zebrafish that led to the identification of PGE 2 as a potent regulator of vertebrate HSC homeostasis.
Zhang, Q. S. et al. Small-molecule synergist of the Wnt–β-catenin signaling pathway. Proc. Natl Acad. Sci. USA 104, 7444–7448 (2007).
Adams, G. B. et al. Therapeutic targeting of a stem cell niche. Nature Biotechnol. 25, 238–243 (2007). This paper provides a proof-of-principle demonstration that targeting stem-cell niches in vivo by conventional therapeutics can enhance stem-cell function and is an attractive strategy for regenerative medicine.
Koprivica, V. et al. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science 310, 106–110 (2005).
Ying, Q.-L. et al. The ground state of embryonic stem cell self-renewal. Nature doi:10.1038/nature06968 (in the press).
Acknowledgements
We thank members of the Ding laboratory for stimulating work and discussions. S.D. is supported by funding from the Scripps Research Institute, the National Institutes of Health (grant numbers MH074404, HD053759, HL084295 and HD058110), the Juvenile Diabetes Research Foundation and the California Institute of Regenerative Medicine.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
S.D. is a founder of Fate Therapeutics.
Additional information
Correspondence should be addressed to S.D. (sding@scripps.edu).
Rights and permissions
About this article
Cite this article
Xu, Y., Shi, Y. & Ding, S. A chemical approach to stem-cell biology and regenerative medicine. Nature 453, 338–344 (2008). https://doi.org/10.1038/nature07042
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07042
This article is cited by
-
In vivo chemical reprogramming of astrocytes into neurons
Cell Discovery (2021)
-
Polyrotaxanes as emerging biomaterials for tissue engineering applications: a brief review
Inflammation and Regeneration (2020)
-
Recent technological advancements in stem cell research for targeted therapeutics
Drug Delivery and Translational Research (2020)
-
CellSim: a novel software to calculate cell similarity and identify their co-regulation networks
BMC Bioinformatics (2019)
-
Pluripotent stem cell-derived organogenesis in the rat model system
Transgenic Research (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.