Abstract
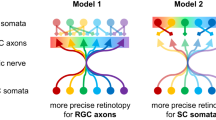
The retinotectal projection has long been studied experimentally and theoretically, as a model for the formation of topographic brain maps1,2,3. Neighbouring retinal ganglion cells (RGCs) project their axons to neighbouring positions in the optic tectum, thus re-establishing a continuous neural representation of visual space. Mapping along this axis requires chemorepellent signalling from tectal cells, expressing ephrin-A ligands, to retinal growth cones, expressing EphA receptors4. High concentrations of ephrin A, increasing from anterior to posterior, prevent temporal axons from invading the posterior tectum. However, the force that drives nasal axons to extend past the anterior tectum and terminate in posterior regions remains to be identified. We tested whether axon–axon interactions, such as competition, are required for posterior tectum innervation. By transplanting blastomeres from a wild-type (WT) zebrafish into a lakritz (lak) mutant, which lacks all RGCs5, we created chimaeras with eyes that contained single RGCs. These solitary RGCs often extended axons into the tectum, where they branched to form a terminal arbor. Here we show that the distal tips of these arbors were positioned at retinotopically appropriate positions, ruling out an essential role for competition in innervation of the ephrin-A-rich posterior tectum. However, solitary arbors were larger and more complex than under normal, crowded conditions, owing to a lack of pruning of proximal branches during refinement of the retinotectal projection. We conclude that dense innervation is not required for targeting of retinal axons within the zebrafish tectum but serves to restrict arbor size and shape.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Goodhill, G. J. Contributions of theoretical modeling to the understanding of neural map development. Neuron 56, 301–311 (2007)
Ruthazer, E. S. & Cline, H. T. Insights into activity-dependent map formation from the retinotectal system: a middle-of-the-brain perspective. J. Neurobiol. 59, 134–146 (2004)
Lemke, G. & Reber, M. Retinotectal mapping: new insights from molecular genetics. Annu. Rev. Cell Dev. Biol. 21, 551–580 (2005)
Flanagan, J. G. Neural map specification by gradients. Curr. Opin. Neurobiol. 16, 59–66 (2006)
Kay, J. N., Finger-Baier, K. C., Roeser, T., Staub, W. & Baier, H. Retinal ganglion cell genesis requires lakritz, a zebrafish atonal homolog. Neuron 30, 725–736 (2001)
Hornberger, M. R. et al. Modulation of EphA receptor function by coexpressed ephrinA ligands on retinal ganglion cell axons. Neuron 22, 731–742 (1999)
Carvalho, R. F. et al. Silencing of EphA3 through a cis interaction with ephrinA5. Nature Neurosci. 9, 322–330 (2006)
Monschau, B. et al. Shared and distinct functions of RAGS and ELF-1 in guiding retinal axons. EMBO J. 16, 1258–1267 (1997)
Sperry, R. W. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc. Natl Acad. Sci. USA 50, 703–710 (1963)
Gierer, A. Model for the retino-tectal projection. Proc. R. Soc. Lond. B 218, 77–93 (1983)
Honda, H. Competition between retinal ganglion axons for targets under the servomechanism model explains abnormal retinocollicular projection of Eph receptor-overexpressing or ephrin-lacking mice. J. Neurosci. 23, 10368–10377 (2003)
Yates, P. A., Holub, A. D., McLaughlin, T., Sejnowski, T. J. & O’Leary, D. D. Computational modeling of retinotopic map development to define contributions of EphA–ephrinA gradients, axon–axon interactions, and patterned activity. J. Neurobiol. 59, 95–113 (2004)
Reber, M., Burrola, P. & Lemke, G. A relative signalling model for the formation of a topographic neural map. Nature 431, 847–853 (2004)
Tsigankov, D. N. & Koulakov, A. A. A unifying model for activity-dependent and activity-independent mechanisms predicts complete structure of topographic maps in ephrin–A deficient mice. J. Comput. Neurosci. 21, 101–114 (2006)
Goodhill, G. J. & Xu, J. The development of retinotectal maps: a review of models based on molecular gradients. Network 16, 5–34 (2005)
Willshaw, D. Analysis of mouse EphA knockins and knockouts suggests that retinal axons programme target cells to form ordered retinotopic maps. Development 133, 2705–2717 (2006)
Fraser, S. E. & Perkel, D. H. Competitive and positional cues in the patterning of nerve connections. J. Neurobiol. 21, 51–72 (1990)
Kay, J. N., Link, B. A. & Baier, H. Staggered cell-intrinsic timing of ath5 expression underlies the wave of ganglion cell neurogenesis in the zebrafish retina. Development 132, 2573–2585 (2005)
Poggi, L., Vitorino, M., Masai, I. & Harris, W. A. Influences on neural lineage and mode of division in the zebrafish retina in vivo. J. Cell Biol. 171, 991–999 (2005)
Xiao, T., Roeser, T., Staub, W. & Baier, H. A GFP-based genetic screen reveals mutations that disrupt the architecture of the zebrafish retinotectal projection. Development 132, 2955–2967 (2005)
Stuermer, C. A. Retinotopic organization of the developing retinotectal projection in the zebrafish embryo. J. Neurosci. 8, 4513–4530 (1988)
Hua, J. Y., Smear, M. C., Baier, H. & Smith, S. J. Regulation of axon growth in vivo by activity-based competition. Nature 434, 1022–1026 (2005)
Smear, M. C. et al. Vesicular glutamate transport at a central synapse limits the acuity of visual perception in zebrafish. Neuron 53, 65–77 (2007)
Baier, H. et al. Genetic dissection of the retinotectal projection. Development 123, 415–425 (1996)
Brennan, C. et al. Two Eph receptor tyrosine kinase ligands control axon growth and may be involved in the creation of the retinotectal map in the zebrafish. Development 124, 655–664 (1997)
Picker, A. et al. Requirement for the zebrafish mid-hindbrain boundary in midbrain polarisation, mapping and confinement of the retinotectal projection. Development 126, 2967–2978 (1999)
Hansen, M. J., Dallal, G. E. & Flanagan, J. G. Retinal axon response to ephrin–As shows a graded, concentration-dependent transition from growth promotion to inhibition. Neuron 42, 717–730 (2004)
Rashid, T. et al. Opposing gradients of ephrin–As and EphA7 in the superior colliculus are essential for topographic mapping in the mammalian visual system. Neuron 47, 57–69 (2005)
von Boxberg, Y., Deiss, S. & Schwarz, U. Guidance and topographic stabilization of nasal chick retinal axons on target-derived components in vitro. Neuron 10, 345–357 (1993)
Ichijo, H. & Bonhoeffer, F. Differential withdrawal of retinal axons induced by a secreted factor. J. Neurosci. 18, 5008–5018 (1998)
Haas, K., Sin, W. C., Javaherian, A., Li, Z. & Cline, H. T. Single-cell electroporation for gene transfer in vivo. Neuron 29, 583–591 (2001)
Acknowledgements
We thank T. Xiao, A. Picker, U. Drescher, K. Haas and H. Cline for reagents and advice, and J. Pinkston-Gosse and members of the Baier laboratory for review of the manuscript. This work was supported by the National Institutes of Health (H.B. and N.J.G.) and a March of Dimes Research Grant (H.B.).
Author Contributions N.J.G., L.M.N. and H.B. devised experiments. N.J.G. performed all transplantation experiments and quantified images. L.M.N. conducted single-cell labelling studies in the tectum. N.J.G. and H.B. wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Video
The file contains Supplementary Video S1 with RGC axon pathfinding errors during retinal exit in WTBrn3c:mGFP-≳ lak chimeras. Dorsal confocal stack of 7 dpf WTBrn3c:mGFP-≳ lak chimera with ˜5 RGCs in varying retinal positions projecting axons. One axon (blue arrow), is visible exiting the retina, but peripheral RGC axons meander and branch in aberrant directions (red arrows). Similar errors were seen in 〈5% of WTBrn3c:mGFP-≳ lak chimeras with one or more axons projecting to the tectum. Orientation: nasal up, temporal down. (MOV 10950 kb)
Supplementary Figures
The file contains Supplementary Figures S2, S3: Example quantification of retinal and tectal position in a single chimeric larva and corresponding legend. Frequency of cell types identified by single cell electroporation in WT and lakritz larvae and corresponding legend. (PDF 19634 kb)
Rights and permissions
About this article
Cite this article
Gosse, N., Nevin, L. & Baier, H. Retinotopic order in the absence of axon competition. Nature 452, 892–895 (2008). https://doi.org/10.1038/nature06816
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature06816
This article is cited by
-
Teleosemantics, selection and novel contents
Biology & Philosophy (2019)
-
Axon-Schwann cell interactions during peripheral nerve regeneration in zebrafish larvae
Neural Development (2014)
-
Branch management: mechanisms of axon branching in the developing vertebrate CNS
Nature Reviews Neuroscience (2014)
-
Restricted perinatal retinal degeneration induces retina reshaping and correlated structural rearrangement of the retinotopic map
Nature Communications (2013)
-
A simple model can unify a broad range of phenomena in retinotectal map development
Biological Cybernetics (2011)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.