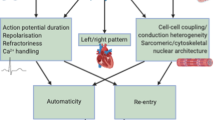
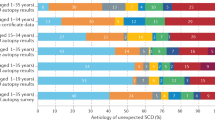
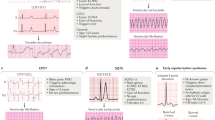
Abstract
Abnormalities in heart rhythm continue to cause high rates of illness and death. Better treatment could be provided by solving two main challenges: the early identification of patients who are at risk, and the characterization of molecular pathways that culminate in arrhythmias. By analysing mechanisms that increase susceptibility to arrhythmia in individuals with genetic syndromes, it might be possible to improve current therapies and to develop new ways to treat and prevent common arrhythmias.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Zheng, Z. J., Croft, J. B., Giles, W. H. & Mensah, G. A. Sudden cardiac death in the United States, 1989 to 1998. Circulation 104, 2158–2163 (2001).
Vreede-Swagemakers, J. J. M. et al. Out-of-hospital cardiac arrest in the 1990s — A population-based study in the Maastricht area on incidence, characteristics and survival. J. Am. Coll. Cardiol. 30, 1500–1505 (1997).
Huikuri, H. V., Castellanos, A. & Myerburg, R. J. Sudden death due to cardiac arrhythmias. N. Engl. J. Med. 345, 1473–1482 (2001).
Page, R. L. & Roden, D. M. Drug therapy for atrial fibrillation: where do we go from here? Nature Rev. Drug Discov. 4, 899–910 (2005).
Nattel, S. & Carlsson, L. Innovative approaches to anti-arrhythmic drug therapy. Nature Rev. Drug Discov. 5, 1034–1049 (2006).
Haissaguerre, M. et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 339, 659–666 (1998).
Darbar, D. et al. Familial atrial fibrillation is a genetically heterogeneous disorder. J. Am. Coll. Cardiol. 41, 2185–2192 (2003).
Fox, C. S. et al. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. J. Am. Med. Assoc. 291, 2851–2855 (2004).
Yao, J. A. et al. Remodeling of gap junctional channel function in epicardial border zone of healing canine infarcts. Circ. Res. 92, 437–443 (2003).
Benjamin, E. J. et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. J. Am. Med. Assoc. 271, 840–844 (1994).
Allessie, M. A. et al. Pathophysiology and prevention of atrial fibrillation. Circulation 103, 769–777 (2001).
Anyukhovsky, E. P. et al. Age-associated changes in electrophysiologic remodeling: a potential contributor to initiation of atrial fibrillation. Cardiovasc. Res. 66, 353–363 (2005).
Spach, M. S. Mounting evidence that fibrosis generates a major mechanism for atrial fibrillation. Circ. Res. 101, 743–745 (2007).
Wijffels, M. C., Kirchhof, C. J., Dorland, R. & Allessie, M. A. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 92, 1954–1968 (1995). This paper was the first to describe the finding that rapid atrial pacing remodels the atrium and thereby predisposes individuals to atrial fibrillation.
Wijffels, M. C., Kirchhof, C. J., Dorland, R., Power, J. & Allessie, M. A. Electrical remodeling due to atrial fibrillation in chronically instrumented conscious goats: roles of neurohumoral changes, ischemia, atrial stretch, and high rate of electrical activation. Circulation 96, 3710–3720 (1997).
Yue, L., Melnyk, P., Gaspo, R., Wang, Z. & Nattel, S. Molecular mechanisms underlying ionic remodeling in a dog model of atrial fibrillation. Circ. Res. 84, 776–784 (1999).
Nattel, S. New ideas about atrial fibrillation 50 years on. Nature 415, 219–226 (2002).
Dobrev, D. & Ravens, U. Remodeling of cardiomyocyte ion channels in human atrial fibrillation. Basic Res. Cardiol. 98, 137–148 (2003).
Dun, W., Chandra, P., Danilo, P. Jr, Rosen, M. R. & Boyden, P. A. Chronic atrial fibrillation does not further decrease outward currents. It increases them. Am. J. Physiol. Heart Circ. Physiol. 285, H1378–H1384 (2003).
Kumagai, K. et al. Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J. Am. Coll. Cardiol. 41, 2197–2204 (2003).
Nakano, Y. et al. Matrix metalloproteinase-9 contributes to human atrial remodeling during atrial fibrillation. J. Am. Coll. Cardiol. 43, 818–825 (2004).
Cardin, S. et al. Contrasting gene expression profiles in two canine models of atrial fibrillation. Circ. Res. 100, 425–433 (2007).
Nerheim, P., Birger-Botkin, S., Piracha, L. & Olshansky, B. Heart failure and sudden death in patients with tachycardia-induced cardiomyopathy and recurrent tachycardia. Circulation 110, 247–252 (2004).
Han, W., Chartier, D., Li, D. & Nattel, S. Ionic remodeling of cardiac Purkinje cells by congestive heart failure. Circulation 104, 2095–2100 (2001).
Akar, F. G., Spragg, D. D., Tunin, R. S., Kass, D. A. & Tomaselli, G. F. Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circ. Res. 95, 717–725 (2004).
Frey, N., Katus, H. A., Olson, E. N. & Hill, J. A. Hypertrophy of the heart: a new therapeutic target? Circulation 109, 1580–1589 (2004).
Schwartz, P. J. The congenital long QT syndromes from genotype to phenotype: clinical implications. J. Intern. Med. 259, 39–47 (2006).
Ashrafian, H. & Watkins, H. Reviews of translational medicine and genomics in cardiovascular disease: new disease taxonomy and therapeutic implications cardiomyopathies: therapeutics based on molecular phenotype. J. Am. Coll. Cardiol. 49, 1251–1264 (2007).
Sen-Chowdhry, S. et al. Clinical and genetic characterization of families with arrhythmogenic right ventricular dysplasia/cardiomyopathy provides novel insights into patterns of disease expression. Circulation 115, 1710–1720 (2007).
Schwartz, P. J., Priori, S. G. & Napolitano, C. How really rare are rare diseases?: the intriguing case of independent compound mutations in the long QT syndrome. J. Cardiovasc. Electrophysiol. 14, 1120–1121 (2003).
Task Force of the Working Group on Arrhythmias of the European Society of Cardiology. The Sicilian Gambit: A new approach to the classification of antiarrhythmic drugs based on their actions on arrhythmogenic mechanisms. Circulation 84, 1831–1851 (1991). The Sicilian Gambit group popularized the idea that antiarrhythmic therapies would be most effective if targeted against specific underlying mechanisms.
Gollob, M. H. et al. Somatic mutations in the connexin 40 gene (GJA5) in atrial fibrillation. N. Engl. J. Med. 354, 2677–2688 (2006). This report raised the intriguing possibility of arrhythmias as a manifestation of somatic mutations rather than germline mutations.
Niimura, H. et al. Mutations in the gene for cardiac myosin-binding protein C and late-onset familial hypertrophic cardiomyopathy. N. Engl. J. Med. 338, 1248–1257 (1998).
Watkins, H. et al. Characteristics and prognostic implications of myosin missense mutations in familial hypertrophic cardiomyopathy. N. Engl. J. Med. 326, 1108–1114 (1992).
Moss, A. J. et al. Clinical aspects of type-1 long-QT syndrome by location, coding type, and biophysical function of mutations involving the KCNQ1 gene. Circulation 115, 2481–2489 (2007).
Crotti, L. et al. The common long-QT syndrome mutation KCNQ1/A341V causes unusually severe clinical manifestations in patients with different ethnic backgrounds. Toward a mutation-specific risk stratification. Circulation 116, 2366–2375 (2007).
Priori, S. G., Napolitano, C. & Schwartz, P. J. Low penetrance in the long-QT syndrome: clinical impact. Circulation 99, 529–533 (1999).
Jouven, X., Desnos, M., Guerot, C. & Ducimetiere, P. Predicting sudden death in the population: the Paris Prospective Study I. Circulation 99, 1978–1983 (1999).
Friedlander, Y. et al. Family history as a risk factor for primary cardiac arrest. Circulation 97, 155–160 (1998).
Dekker, L. R. C. et al. Familial sudden death is an important risk factor for primary ventricular fibrillation: A case-control study in acute myocardial infarction patients. Circulation 114, 1140–1145 (2006). Refs 37–40 identify a family history of SCD as a potent risk factor for SCD.
Splawski, I. et al. Variant of SCN5A sodium channel implicated in risk of cardiac arrhythmia. Science 297, 1333–1336 (2002).
Ellinor, P. T. & Macrae, C. A. The genetics of atrial fibrillation. J. Cardiovasc. Electrophysiol. 14, 1007–1009 (2003).
Darbar, D., Hardy, A., Haines, J. L. & Roden, D. M. A novel locus on chromosome 5 for familial atrial fibrillation associated with prolonged signal-averaged p-wave. J. Am. Coll. Cardiol. (in the press).
Gudbjartsson, D. F. et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature 448, 353–357 (2007).
Mommersteeg, M. T. M. et al. Pitx2c and Nkx2-5 are required for the formation and identity of the pulmonary myocardium. Circ. Res. 101, 902–909 (2007).
Arking, D. E. et al. A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nature Genet. 38, 644–651 (2006).
Aarnoudse, A. J. et al. Common NOS1AP variants are associated with a prolonged QTc interval in the Rotterdam study. Circulation 116, 10–16 (2007).
Post, W. et al. Associations between genetic variants in the NOS1AP (CAPON) gene and cardiac repolarization in the Old Order Amish. Hum. Hered. 64, 214–219 (2007).
Lehnart, S. E. et al. Inherited arrhythmias: A National Heart, Lung, and Blood Institute and Office of Rare Diseases workshop consensus report about the diagnosis, phenotyping, molecular mechanisms, and therapeutic approaches for primary cardiomyopathies of gene mutations affecting ion channel function. Circulation 116, 2325–2345 (2007).
Lehnart, S. & Marks, A. R. Regulation of ryanodine receptors in the heart. Circ. Res. 101, 746–749 (2007).
Priori, S. G. et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 103, 196–200 (2001).
Lahat, H. et al. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am. J. Hum. Genet. 69, 1378–1384 (2001).
Cerrone, M. et al. Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor (RyR2). Circ. Res. 96, e77–e82 (2005).
Knollmann, B. C. et al. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J. Clin. Invest. 116, 2510–2520 (2006).
Chopra, N. et al. Modest reductions of cardiac calsequestrin increase sarcoplasmic reticulum Ca2+ leak independent of luminal Ca2+ and trigger ventricular arrhythmias in mice. Circ. Res. 101, 617–626 (2007).
Vest, J. A. et al. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation 111, 2025–2032 (2005).
Wehrens, X. H. et al. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc. Natl Acad. Sci. USA 103, 511–518 (2006).
Marx, S. O. et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101, 365–376 (2000).
Reiken, S. R. et al. Protein kinase A phosphorylation of the cardiac calcium release channel (ryanodine receptor) in normal and failing hearts: role of phosphatases and response to isoproterenol. J. Biol. Chem. 278, 444–453 (2002).
Wehrens, X. H. et al. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science 304, 292–296 (2004).
Benkusky, N. A. et al. Intact beta-adrenergic response and unmodified progression toward heart failure in mice with genetic ablation of a major protein kinase A phosphorylation site in the cardiac ryanodine receptor. Circ. Res. 101, 819–829 (2007).
Xiao, J. et al. Removal of FKBP12.6 does not alter the conductance and activation of cardiac ryanodine receptor and the susceptibility to stress-induced ventricular arrhythmias. J. Biol. Chem. 282, 34828–34838 (2007).
Hunt, D. J. et al. K201 (JTV519) suppresses spontaneous Ca2+ release and [3H]ryanodine binding to RyR2 irrespective of FKBP12.6 association. Biochem. J. 404, 431–438 (2007).
Spirito, P., Seidman, C. E., McKenna, W. J. & Maron, B. J. The management of hypertrophic cardiomyopathy. N. Engl. J. Med. 336, 775–785 (1997).
Knollmann, B. C. et al. Familial hypertrophic cardiomyopathy-linked mutant troponin T causes stress-induced ventricular tachycardia and Ca2+-dependent action potential remodeling. Circ. Res. 92, 428–436 (2003).
Sirenko, S.G., Potter, J. D. & Knollmann, B. C. Differential effect of troponin T mutations on the inotropic responsiveness of mouse hearts — role of myofilament Ca2+ sensitivity increase. J. Physiol. 575, 201–213 (2006).
Ai, X., Curran, J. W., Shannon, T. R., Bers, D. M. & Pogwizd, S. M. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ. Res. 97, 1314–1322 (2005).
Curran, J., Hinton, M. J., Rios, E., Bers, D. M. & Shannon, T. R. Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ. Res. 100, 391–398 (2007).
Anderson, M. E. et al. KN-93, an inhibitor of multifunctional Ca2+/calmodulin dependent protein kinase, decreases early afterdepolarizations in rabbit heart. J. Pharmacol. Exp.Ther. 287, 996–1006 (1998).
Mazur, A., Roden, D. M. & Anderson, M. E. Systemic administration of calmodulin antagonist W-7 or protein kinase A inhibitor H-8 prevents torsade de pointes in rabbits. Circulation 100, 2437–2442 (1999).
Bers, D. M. Beyond beta blockers. Nature Med. 11, 379–380 (2005).
Zhang, R. et al. Calmodulin kinase II inhibition protects against structural heart disease. Nature Med. 11, 409–417 (2005). This paper reports that transgenic mice expressing a CaMKII-inhibitory peptide were protected against post-myocardial-infarction remodelling and arrhythmias.
Khoo, M. S. et al. Death, cardiac dysfunction and arrhythmias due to up-regulation of calmodulin kinase II in calcineurin-induced cardiomyopathy. Circulation 114, 1352–1359 (2006).
Sasano, T., McDonald, A. D., Kikuchi, K. & Donahue, J. K. Molecular ablation of ventricular tachycardia after myocardial infarction. Nature Med. 12, 1256–1258 (2006).
Bucchi, A. et al. Wild-type and mutant HCN channels in a tandem biological-electronic cardiac pacemaker. Circulation 114, 992–999 (2006).
Mcanulty, J. et al. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N. Engl. J. Med. 337, 1576–1583 (1997).
Moss, A. J. et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N. Engl. J. Med. 346, 877–883 (2002). This was the first report from a large trial showing that prophylactic implantation of ICDs in patients at high risk for future cardiac arrest reduces mortality.
Bardy, G. H. et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 352, 225–237 (2005).
Spooner, P. M. et al. Sudden cardiac death, genes, and arrhythmogenesis: consideration of new population and mechanistic approaches from a national heart, lung, and blood institute workshop, part I. Circulation 103, 2361–2364 (2001).
Young, J. B. et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD trial. J. Am. Med. Assoc. 289, 2685–2694 (2003).
The CAST Investigators. Preliminary report: Effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N. Engl. J. Med. 321, 406–412 (1989). This report shows that Na+-channel-blocking drugs increase mortality in patients convalescing from acute myocardial infarction.
Krishnan, S.C. & Antzelevitch, C. Flecainide-induced arrhythmia in canine ventricular epicardium. Phase 2 reentry? Circulation 87, 562–572 (1993).
Lukas, A. & Antzelevitch, C. Differences in the electrophysiological response of canine ventricular epicardium and endocardium to ischemia. Role of the transient outward current. Circulation 88, 2903–2915 (1993).
Coromilas, J., Saltman, A. E., Waldecker, B., Dillon, S. M. & Wit, A. L. Electrophysiological effects of flecainide on anisotropic conduction and reentry in infarcted canine hearts. Circulation 91, 2245–2263 (1995).
Waldo, A. L. et al. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. Lancet 348, 7–12 (1996).
Kober, L. et al. Effect of dofetilide in patients with recent myocardial infarction and left-ventricular dysfunction: a randomised trial. Danish Investigations of Arrhythmia and Mortality on Dofetilide (DIAMOND) Study Group. Lancet 356, 2052–2058 (2000).
Camm, A. J. et al. Mortality in patients after a recent myocardial infarction: A randomized, placebo-controlled trial of azimilide using heart rate variability for risk stratification. Circulation 109, 990–996 (2004).
Roden, D. M. Drug-induced prolongation of the QT interval. N. Engl. J. Med. 350, 1013–1022 (2004).
Norwegian Multicenter Study Group. Timolol-induced reduction in mortality in reinfarction in patients surviving acute myocardial infarction. N. Engl. J. Med. 304, 801–807 (1981).
Beta-Blocker Heart Attack Trial Research Group. A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. J. Am. Med. Assoc. 247, 1707–1714 (1982).
Murray, K. T., Mace, L. C. & Yang, Z. Nonantiarrhythmic drug therapy for atrial fibrillation. Heart Rhythm. 4, S88–S90 (2007).
Li, D. et al. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation 104, 2608–2614 (2001).
Pedersen, O. D., Bagger, H., Kober, L. & Torp-Pedersen, C. Trandolapril reduces the incidence of atrial fibrillation after acute myocardial infarction in patients with left ventricular dysfunction. Circulation 100, 376–380 (1999).
Vermes, E. et al. Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction. Insight from the studies of left ventricular dysfunction (SOLVD) trials. Circulation 107, 2926–2931 (2003).
Kumagai, K., Nakashima, H. & Saku, K. The HMG-CoA reductase inhibitor atorvastatin prevents atrial fibrillation by inhibiting inflammation in a canine sterile pericarditis model. Cardiovasc. Res. 62, 105–111 (2004).
Shiroshita-Takeshita, A., Schram, G., Lavoie, J. & Nattel, S. Effect of simvastatin and antioxidant vitamins on atrial fibrillation promotion by atrial-tachycardia remodeling in dogs. Circulation 110, 2313–2319 (2004).
Marchioli, R. et al. Early protection against sudden death by n–3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation 105, 1897–1903 (2002). The greatest reduction in the incidence of SCD in a large randomized clinical trial has been with fish oil (53% reduction at 4 months).
Leaf, A., Kang, J. X., Xiao, Y. F. & Billman, G. E. Clinical prevention of sudden cardiac death by n–3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n–3 fish oils. Circulation 107, 2646–2652 (2003).
Sarrazin, J. F. et al. Reduced incidence of vagally induced atrial fibrillation and expression levels of connexins by n–3 polyunsaturated fatty acids in dogs. J. Am. Coll. Cardiol. 50, 1505–1512 (2007).
Morady, F. Radio-frequency ablation as treatment for cardiac arrhythmias. N. Engl. J. Med. 340, 534–544 (1999).
Acknowledgements
This work was supported in part by grants from the United States Public Health Service.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
D.M.R. accepted fees for one-time consultations on QT prolongation by new non-cardiovascular therapies, on genetic testing for new drugs, and/or on developing new antiarrhythmic drugs from Sapphire Therapeutics, Atlas Venture Advisors, Pfizer, Avanir Pharmaceuticals, Baker Brothers Advisors, CardioKine and Eli Lilly and Company. D.M.R. also is paid royalties on a patent on D85N as a predictive single-nucleotide polymorphism for drug-induced long-QT syndrome. B.C.K. declares no competing interests.
Additional information
Correspondence should be addressed to D.M.R. (dan.roden@vanderbilt.edu).
Rights and permissions
About this article
Cite this article
Knollmann, B., Roden, D. A genetic framework for improving arrhythmia therapy. Nature 451, 929–936 (2008). https://doi.org/10.1038/nature06799
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature06799
This article is cited by
-
Microengineered platforms for characterizing the contractile function of in vitro cardiac models
Microsystems & Nanoengineering (2022)
-
Pathophysiology, Prevention, and Treatment of Commotio Cordis
Current Cardiology Reports (2014)
-
Calsequestrin Mutations and Catecholaminergic Polymorphic Ventricular Tachycardia
Pediatric Cardiology (2012)
-
Heart failure: advances through genomics
Nature Reviews Genetics (2011)
-
Genetic variation in SCN10A influences cardiac conduction
Nature Genetics (2010)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.